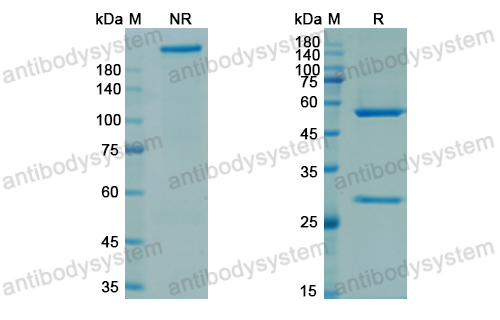
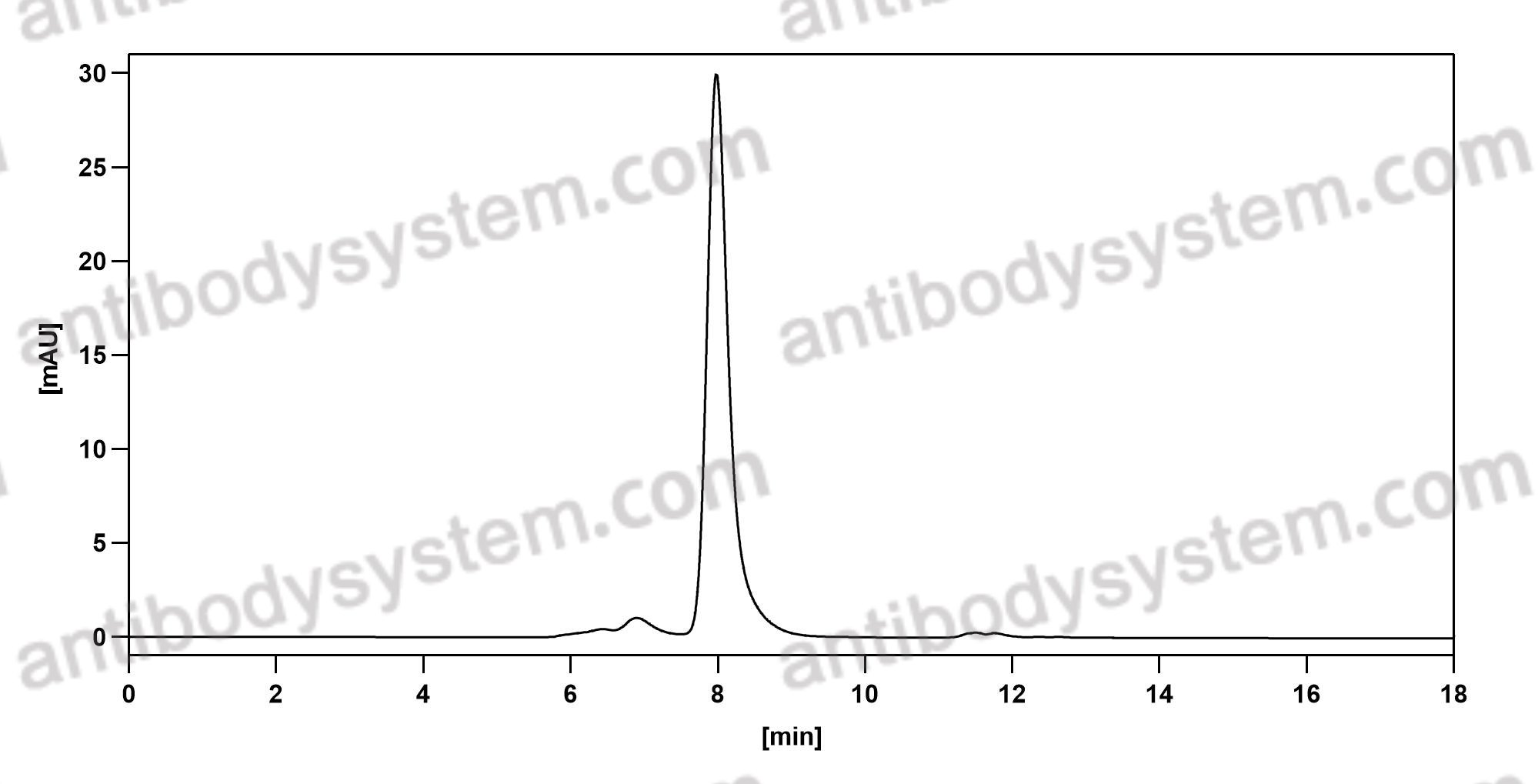
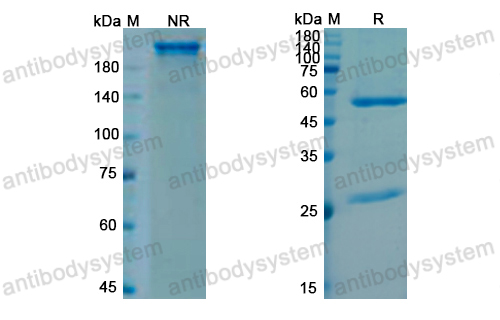
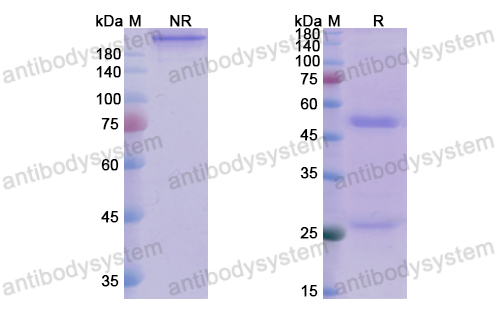
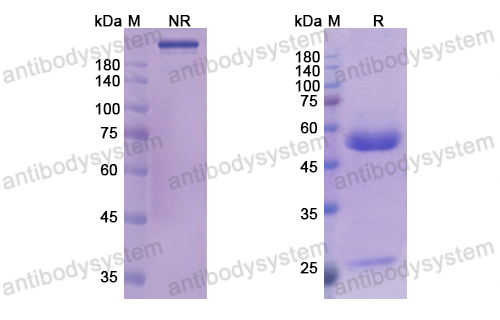
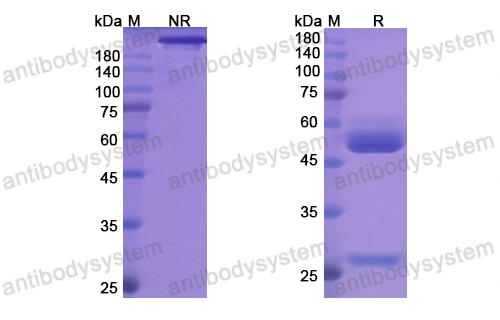
Efficacy and Safety of Rozanolixizumab in Moderate to Severe Generalized Myasthenia Gravis: A Phase 2 Randomized Control Trial, PMID: 33219142
The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study, PMID: 29093180
Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration, PMID: 30130439
Phase 2 multiple-dose study of an FcRn inhibitor, rozanolixizumab, in patients with primary immune thrombocytopenia, PMID: 32886753
Next-generation Fc receptor-targeting biologics for autoimmune diseases, PMID: 31404703
Progress in the therapy of myasthenia gravis: getting closer to effective targeted immunotherapies, PMID: 32833750
Autoimmune thrombocytopenia: Current treatment options in adults with a focus on novel drugs, PMID: 31449692
Current pharmacotherapeutic options for myasthenia gravis, PMID: 31670984
Erratum for the Research Article: "The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study" by P. Kiessling, R. Lledo-Garcia, S. Watanabe, G. Langdon, D. Tran, M. Bari, L. Christodoulou, E. Jones, G. Price, B. Smith, F. Brennan, I. White, S. Jolles, PMID: 29212714
Therapies Directed Against B-Cells and Downstream Effectors in Generalized Autoimmune Myasthenia Gravis: Current Status, PMID: 30762205
[Reaserch Advances in the Treatment of Primary Immune Thrombocytopenia--Review], PMID: 34105504
Development and Evaluation of a Physiologically Based Pharmacokinetic Model for Predicting the Effects of Anti-FcRn Therapy on the Disposition of Endogenous IgG in Humans, PMID: 30471293
The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors, PMID: 33717213
Antiseizure Medication use in Gastric Bypass Patients and Other Post-Surgical Malabsorptive States, PMID: 33997757
FcRn inhibitors in immune thrombocytopenia: A comprehensive review of therapeutic advances and clinical outcomes., PMID:40505332
From checkboxes to emojis: a novel approach to patient-reported outcomes in multiple sclerosis., PMID:40447786
Assessing fall risk in multiple sclerosis using patient-reported outcomes and wearable gait metrics., PMID:40292035
Efficacy and safety of FcRn inhibitors in patients with Myasthenia gravis: An updated systematic review and meta‑analysis., PMID:40288289
Rozanolixizumab for Myasthenia Gravis: a breakthrough treatment and future prospects., PMID:40277145
Real-World Experience with FcRn Inhibitors Efgartigimod and Rozanolixizumab in Myasthenia Gravis: Administration in Multiple Cycles and Transition from Intravenous to Subcutaneous Formulation., PMID:40257679
Drug-induced herpes zoster: a pharmacovigilance analysis of FDA adverse event reports from 2004 to 2024., PMID:40206093
Rising to the challenge: an international Delphi consensus study on fetal and neonatal alloimmune thrombocytopenia., PMID:40175002
Ventral Subpial and Central Cord Enhancement in Spinal Neurosarcoidosis: The Reverse Trident Sign., PMID:40168633
Safety and efficacy of chronic weekly rozanolixizumab in generalized myasthenia gravis: the randomized open-label extension MG0004 study., PMID:40105996
Long-term safety of cyclical rozanolixizumab in patients with generalized myasthenia gravis: Results from the Phase 3 MycarinG study and an open-label extension., PMID:40034001
Rozanolixizumab in generalized myasthenia gravis: Pooled analysis of the Phase 3 MycarinG study and two open-label extensions., PMID:40033991
Assessment of concurrent neoplasms and a paraneoplastic association in MOGAD., PMID:39932929
Real-world clinical experience with serum MOG and AQP4 antibody testing by live versus fixed cell-based assay., PMID:39901660
FcRn inhibitors in the context of myasthenia gravis., PMID:39870595
Testing for myelin oligodendrocyte glycoprotein antibodies: Who, what, where, when, why, and how., PMID:39861933
Curriculum Innovations: A Novel Neurology Clinician-Educator Program., PMID:39748890
Evaluation of the effect of rozanolixizumab on pregnancy outcomes and pre- and postnatal development in cynomolgus monkeys., PMID:39709064
Advances in Disease-Modifying Therapeutics for Chronic Neuromuscular Disorders., PMID:39708835
Autoimmune brainstem encephalitis: Clinical associations, outcomes, and proposed diagnostic criteria., PMID:39708293
Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Neonatal Fc Receptor Inhibitor Rozanolixizumab: An Ethnic Sensitivity Study in Healthy Japanese, Chinese, and White Participants., PMID:39569616
Inhibition of FcRn with rozanolixizumab in adults with immune thrombocytopenia: Two randomised, double-blind, placebo-controlled phase 3 studies and their open-label extension., PMID:39552477
Initiation response, maximized therapeutic efficacy, and post-treatment effects of biological targeted therapies in myasthenia gravis: a systematic review and network meta-analysis., PMID:39529623
Risk-Benefit Analysis of Novel Treatments for Patients with Generalized Myasthenia Gravis., PMID:39470879
Pediatric MOG-Ab-Associated Encephalitis: Supporting Early Recognition and Treatment., PMID:39393046
Comprehensive Analysis of Paraneoplastic Neurologic Syndrome and PNS-CARE Diagnostic Criteria in Clinical Practice., PMID:39321395
Efficacy and safety of rozanolixizumab in patients with muscle-specific tyrosine kinase autoantibody-positive generalised myasthenia gravis: a subgroup analysis of the randomised, double-blind, placebo-controlled, adaptive phase III MycarinG study., PMID:39297052
Intracranial Pressure Elevation and MOGAD., PMID:39283648
Long-term outcomes in antibody-negative autoimmune encephalitis: a retrospective study., PMID:39278895
Blood neurofilament light chain measurements in adults with CNS histiocytic neoplasms., PMID:39237493
Targeted Treatments for Myasthenia Gravis in Children and Adolescents., PMID:39198371
Application of the international criteria for optic neuritis in the Acute Optic Neuritis Network., PMID:39099240
Progress report on new medications for seizures and epilepsy: A summary of the 17th Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XVII). I. Drugs in preclinical and early clinical development., PMID:39008349
Clinical meaningfulness and psychometric robustness of the MG Symptoms PRO scales in clinical trials in adults with myasthenia gravis., PMID:38978809
Efgartigimod as Rescue Medication in a Patient with Therapy-Refractory Myasthenic Crisis., PMID:38939234
Efficacy, safety and tolerability of rozanolixizumab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a randomised, subject-blind, investigator-blind, placebo-controlled, phase 2a trial and open-label extension study., PMID:38729747
FcRn Inhibitor Therapies in Neurologic Diseases., PMID:38724842
MOG Antibody-Associated Disease in the Setting of Metastatic Melanoma Complicated by Immune Checkpoint Inhibitor Use., PMID:38696737
Improving the sensitivity of myelin oligodendrocyte glycoprotein-antibody testing: exclusive or predominant MOG-IgG3 seropositivity-a potential diagnostic pitfall in patients with MOG-EM/MOGAD., PMID:38609667
Zilucoplan (Zilbrysq) for myasthenia gravis., PMID:38576149
Rozanolixizumab: A New Therapy in the Treatment of Myasthenia Gravis., PMID:38533739
Clinical and neuroimaging phenotypes of autoimmune glial fibrillary acidic protein astrocytopathy: A systematic review and meta-analysis., PMID:38506182
Batoclimab vs Placebo for Generalized Myasthenia Gravis: A Randomized Clinical Trial., PMID:38436998
The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis., PMID:38431900
Rozanolixizumab (Rystiggo) for myasthenia gravis., PMID:38412267
The Efficacy and Safety of Different Targeted Drugs for the Treatment of Generalized Myasthenia Gravis: A Systematic Review and Bayesian Network Meta-analysis., PMID:38300476
Interactions of the anti-FcRn monoclonal antibody, rozanolixizumab, with Fcγ receptors and functional impact on immune cells in vitro., PMID:38241085
Peripheral nervous system manifestations of MOG antibody associated disease., PMID:38234084
Myasthenia Gravis Treatment: From Old Drugs to Innovative Therapies with a Glimpse into the Future., PMID:38212553
Antibodies to watch in 2024., PMID:38178784