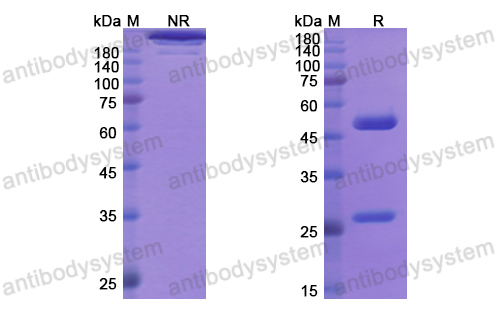
Catalog No.
DHB94503
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
LeIF D, Interferon alpha-1/13, IFNA1, Interferon alpha-D, IFN-alpha-1/13
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P01562
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
RhuMAB IFNalpha, CAS: 948570-30-7
Clone ID
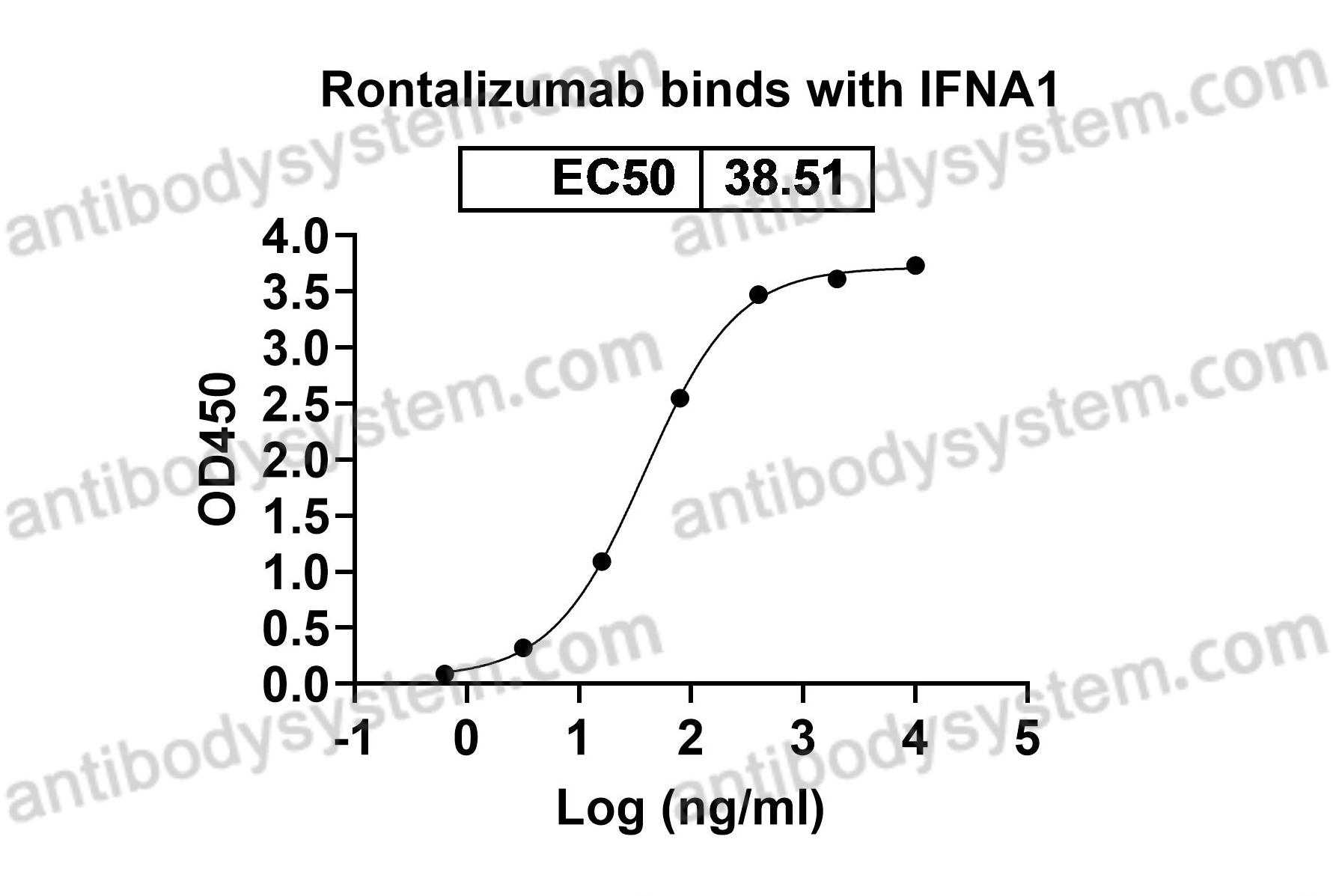
Rontalizumab
A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE), PMID: 26038091
Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations, PMID: 32237942
Biologics targeting type I interferons in SLE: A meta-analysis and systematic review of randomised controlled trials, PMID: 32960720
Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus, PMID: 27270345
From mechanism to therapies in systemic lupus erythematosus, PMID: 28118202
Interferon targeted therapies in systemic lupus erythematosus, PMID: 32185249
Interferon α-targeted therapy, PMID: 23994795
Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells?, PMID: 27497254
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID: 28765121
S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases, PMID: 33748735
Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study, PMID: 22833362
Structural basis of the broadly neutralizing anti-interferon-α antibody rontalizumab, PMID: 26099203
Targeting interferons in systemic lupus erythematosus: current and future prospects, PMID: 25940912
Type I interferon blockade in systemic lupus erythematosus: where do we stand?, PMID: 24344319
Update on clinical trials in systemic lupus erythematosus, PMID: 27314466
S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases., PMID:33748735
Biologics targeting type I interferons in SLE: A meta-analysis and systematic review of randomised controlled trials., PMID:32960720
Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations., PMID:32237942
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID:28765121
Interferon targeted therapies in systemic lupus erythematosus., PMID:32185249
From mechanism to therapies in systemic lupus erythematosus., PMID:28118202
Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells?, PMID:27497254
Update on clinical trials in systemic lupus erythematosus., PMID:27314466
Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus., PMID:27270345
Structural basis of the broadly neutralizing anti-interferon-α antibody rontalizumab., PMID:26099203
A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE)., PMID:26038091
Targeting interferons in systemic lupus erythematosus: current and future prospects., PMID:25940912
Type I interferon blockade in systemic lupus erythematosus: where do we stand?, PMID:24344319
Interferon α-targeted therapy., PMID:23994795
Safety and pharmacodynamics of rontalizumab in patients with systemic lupus erythematosus: results of a phase I, placebo-controlled, double-blind, dose-escalation study., PMID:22833362