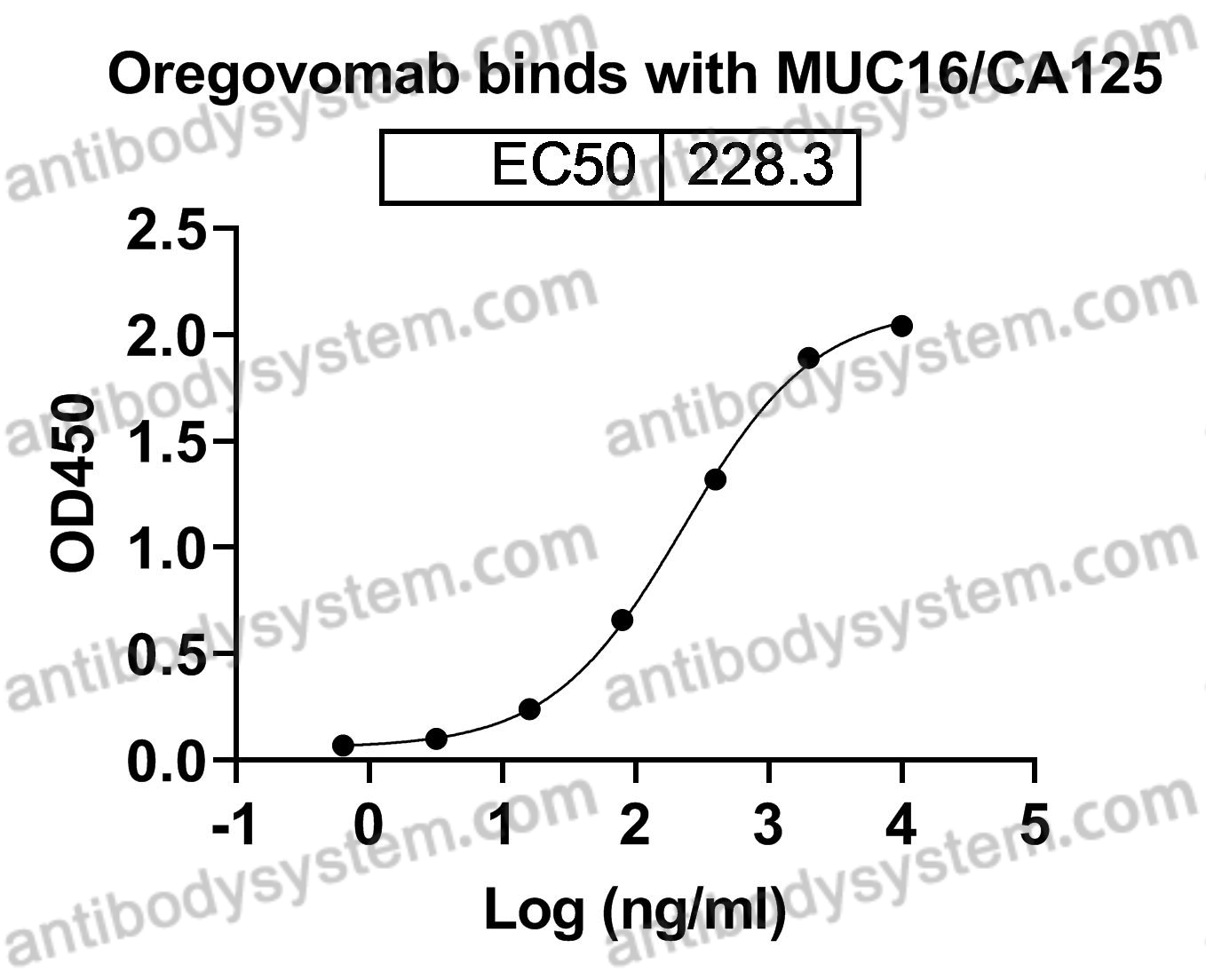
Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: A randomized phase II study, PMID: 31916979
Oregovomab: an investigational agent for the treatment of advanced ovarian cancer, PMID: 33423551
Oregovomab: anti-CA-125 monoclonal antibody B43.13--AltaRex, B43.13, MAb B43.13, monoclonal antibody B43.13, PMID: 17073521
Immunotherapy of ovarian cancer with antibodies: a focus on oregovomab, PMID: 15268682
Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer, PMID: 19075271
Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients, PMID: 31897661
Phase I/II Trial of Neoadjuvant Oregovomab-based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir For Locally Advanced Pancreatic Adenocarcinoma, PMID: 31513018
Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer, PMID: 15337799
Correction to: Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients, PMID: 32219502
CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients, PMID: 15297171
A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer, PMID: 16343178
The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer, PMID: 19307994
CA125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5-year follow-up survey to a randomized placebo-controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer, PMID: 18481390
Antibody-based immunotherapy for ovarian cancer: where are we at?, PMID: 24285017
The antibody-based CA125-targeted maintenance therapy for the epithelial ovarian cancer: a meta-analysis, PMID: 29894066
Monoclonal antibodies in gynecological cancer: a critical point of view, PMID: 22235224
Antibody-based immunotherapy of solid cancers: progress and possibilities, PMID: 26314410
Monoclonal antibody therapy of ovarian cancer, PMID: 15757441
Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy, PMID: 26527287
Gateways to clinical trials, PMID: 17344945
Molecular/genetic therapies in ovarian cancer: future opportunities and challenges, PMID: 22343235
Potential targets for ovarian clear cell carcinoma: a review of updates and future perspectives, PMID: 26675567
The use of monoclonal antibodies for the treatment of epithelial ovarian cancer (review), PMID: 18497976
Gateways to clinical trials, PMID: 12851663
Preclinical studies and clinical utilization of monoclonal antibodies in epithelial ovarian cancer, PMID: 19232697
Biologic and immunologic therapies for ovarian cancer, PMID: 12743131
Gateways to clinical trials, PMID: 15319815
Comparative efficacy of targeted maintenance therapy for newly diagnosed epithelial ovarian cancer: a network meta-analysis, PMID: 31190984
Gateways to clinical trials, PMID: 16810345
Current approaches in ovarian cancer vaccines, PMID: 15729204
Is tissue CA125 expression in epithelial ovarian adenocarcinoma heterogenic?, PMID: 23030299
Gateways to clinical trials. March 2003, PMID: 12731460
Radiation therapy and biological compounds for consolidation therapy in advanced ovarian cancer, PMID: 18336400
New agents and new formulations for the treatment of ovarian cancer, PMID: 16491624
Systematic review update of observational studies further supports aspirin role in cancer treatment: Time to share evidence and decision-making with patients?, PMID: 30252883
OVAREX MAb-B43.13:IFN-gamma could improve the ovarian tumor cell sensitivity to CA125-specific allogenic cytotoxic T cells, PMID: 9085127
Development and characterization of a bispecific single-chain antibody directed against T cells and ovarian carcinoma, PMID: 10768839
Pharmacokinetics and radiation dosimetry of 99Tcm-labelled monoclonal antibody B43.13 in ovarian cancer patients, PMID: 9352556
Immunotherapy of human ovarian carcinoma with OvaRex MAb-B43.13 in a human-PBL-SCID/BG mouse model, PMID: 10211788
Antiidiotype induction therapy: evidence for the induction of immune response through the idiotype network in patients with ovarian cancer after administration of anti-CA125 murine monoclonal antibody B43.13, PMID: 7590780
The effects of circulating antigen on the pharmacokinetics and radioimmunoscintigraphic properties of 99m Tc labelled monoclonal antibodies in cancer patients, PMID: 10948399
Induction of anti-idiotypic humoral and cellular immune responses by a murine monoclonal antibody recognizing the ovarian carcinoma antigen CA125 encapsulated in biodegradable microspheres, PMID: 9755874
Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13--evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo, PMID: 11471484
An antibody-lectin sandwich assay for the determination of CA125 antigen in ovarian cancer patients, PMID: 8872106
Immunohistochemical characterization of 22 monoclonal antibodies against the CA125 antigen: 2nd report from the ISOBM TD-1 Workshop, PMID: 8938947
OPERA: a phase II trial of oregovomab plus non-platinum chemotherapy in PARP inhibitor/platinum-resistant ovarian cancer., PMID:38940373
Front-line chemoimmunotherapy for treating epithelial ovarian cancer: Part II promising results of phase 2 study of paclitaxel-carboplatin-oregovomab regimen., PMID:38216242
Front-line chemo-immunotherapy for treating epithelial ovarian cancer: Part I CA125 and anti-CA125., PMID:38008497
Oregovomab Plus Chemo in Newly Diagnosed Patients with Advanced Epithelial Ovarian Cancer Following Optimal Debulking Surgery (FLORA-5/GOG-3035)., PMID:36400892
Oregovomab: an investigational agent for the treatment of advanced ovarian cancer., PMID:33423551
Correction to: Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients., PMID:32219502
Front-line chemo-immunotherapy with carboplatin-paclitaxel using oregovomab indirect immunization in advanced ovarian cancer: A randomized phase II study., PMID:31916979
Translational immune correlates of indirect antibody immunization in a randomized phase II study using scheduled combination therapy with carboplatin/paclitaxel plus oregovomab in ovarian cancer patients., PMID:31897661
Phase I/II Trial of Neoadjuvant Oregovomab-based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir For Locally Advanced Pancreatic Adenocarcinoma., PMID:31513018
Comparative efficacy of targeted maintenance therapy for newly diagnosed epithelial ovarian cancer: a network meta-analysis., PMID:31190984
Systematic review update of observational studies further supports aspirin role in cancer treatment: Time to share evidence and decision-making with patients?, PMID:30252883
The antibody-based CA125-targeted maintenance therapy for the epithelial ovarian cancer: a meta-analysis., PMID:29894066
Potential targets for ovarian clear cell carcinoma: a review of updates and future perspectives., PMID:26675567
Understanding the Unique Attributes of MUC16 (CA125): Potential Implications in Targeted Therapy., PMID:26527287
Antibody-based immunotherapy of solid cancers: progress and possibilities., PMID:26314410
Antibody-based immunotherapy for ovarian cancer: where are we at?, PMID:24285017
Is tissue CA125 expression in epithelial ovarian adenocarcinoma heterogenic?, PMID:23030299
Molecular/genetic therapies in ovarian cancer: future opportunities and challenges., PMID:22343235
Monoclonal antibodies in gynecological cancer: a critical point of view., PMID:22235224
The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer., PMID:19307994
Preclinical studies and clinical utilization of monoclonal antibodies in epithelial ovarian cancer., PMID:19232697
Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer., PMID:19075271
The use of monoclonal antibodies for the treatment of epithelial ovarian cancer (review)., PMID:18497976
CA125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5-year follow-up survey to a randomized placebo-controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer., PMID:18481390
Radiation therapy and biological compounds for consolidation therapy in advanced ovarian cancer., PMID:18336400
Gateways to clinical trials., PMID:17344945
Oregovomab: anti-CA-125 monoclonal antibody B43.13--AltaRex, B43.13, MAb B43.13, monoclonal antibody B43.13., PMID:17073521
Gateways to clinical trials., PMID:16810345
New agents and new formulations for the treatment of ovarian cancer., PMID:16491624
A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer., PMID:16343178
Monoclonal antibody therapy of ovarian cancer., PMID:15757441
Current approaches in ovarian cancer vaccines., PMID:15729204
Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer., PMID:15337799
Gateways to clinical trials., PMID:15319815
CA125- and tumor-specific T-cell responses correlate with prolonged survival in oregovomab-treated recurrent ovarian cancer patients., PMID:15297171
Immunotherapy of ovarian cancer with antibodies: a focus on oregovomab., PMID:15268682
Gateways to clinical trials., PMID:12851663
Biologic and immunologic therapies for ovarian cancer., PMID:12743131
Gateways to clinical trials. March 2003., PMID:12731460
Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13--evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo., PMID:11471484
The effects of circulating antigen on the pharmacokinetics and radioimmunoscintigraphic properties of 99m Tc labelled monoclonal antibodies in cancer patients., PMID:10948399
Development and characterization of a bispecific single-chain antibody directed against T cells and ovarian carcinoma., PMID:10768839
Immunotherapy of human ovarian carcinoma with OvaRex MAb-B43.13 in a human-PBL-SCID/BG mouse model., PMID:10211788
Induction of anti-idiotypic humoral and cellular immune responses by a murine monoclonal antibody recognizing the ovarian carcinoma antigen CA125 encapsulated in biodegradable microspheres., PMID:9755874
Pharmacokinetics and radiation dosimetry of 99Tcm-labelled monoclonal antibody B43.13 in ovarian cancer patients., PMID:9352556
OVAREX MAb-B43.13:IFN-gamma could improve the ovarian tumor cell sensitivity to CA125-specific allogenic cytotoxic T cells., PMID:9085127
An antibody-lectin sandwich assay for the determination of CA125 antigen in ovarian cancer patients., PMID:8872106
Immunohistochemical characterization of 22 monoclonal antibodies against the CA125 antigen: 2nd report from the ISOBM TD-1 Workshop., PMID:8938947
Antiidiotype induction therapy: evidence for the induction of immune response through the idiotype network in patients with ovarian cancer after administration of anti-CA125 murine monoclonal antibody B43.13., PMID:7590780