Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial, PMID: 27918764
Light-Induced Radiosynthesis of 89 Zr-DFO-Azepin-Onartuzumab for Imaging the Hepatocyte Growth Factor Receptor, PMID: 31924725
The efficacy and safety of onartuzumab in patients with solid cancers: A meta-analysis of randomized trials, PMID: 33402588
Onartuzumab in lung cancer: the fall of Icarus?, PMID: 25818471
Placental and Fetal Effects of Onartuzumab, a Met/HGF Signaling Antagonist, When Administered to Pregnant Cynomolgus Monkeys, PMID: 29893934
89 Zr-Onartuzumab PET imaging of c-MET receptor dynamics, PMID: 28315949
Onartuzumab ineffective in non-small-cell lung cancer, PMID: 28017663
[A new drug in thoracic oncology: MetMab (onartuzumab)], PMID: 23477747
89 Zr-Desferrioxamine p-isothiocyanatobenzyl-anti-hepatocyte growth factor receptor 1-armed antibody onartuzumab, PMID: 23534083
First-line onartuzumab plus erlotinib treatment for patients with MET-positive and EGFR mutation-positive non-small-cell lung cancer, PMID: 30472556
Combining onartuzumab with erlotinib inhibits growth of non-small cell lung cancer with activating EGFR mutations and HGF overexpression, PMID: 25522765
Onartuzumab with or without bevacizumab in combination with weekly paclitaxel does not prolong QTc or adversely affect other ECG parameters in patients with locally recurrent or metastatic triple-negative breast cancer, PMID: 25542267
A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction, PMID: 27401892
Onartuzumab (MetMAb): using nonclinical pharmacokinetic and concentration-effect data to support clinical development, PMID: 23894056
Immuno-PET of the hepatocyte growth factor receptor Met using the 1-armed antibody onartuzumab, PMID: 22917884
Safety of Onartuzumab in Patients with Solid Tumors: Experience to Date from the Onartuzumab Clinical Trial Program, PMID: 26445503
Efficacy and Safety of Onartuzumab in Combination With First-Line Bevacizumab- or Pemetrexed-Based Chemotherapy Regimens in Advanced Non-Squamous Non-Small-Cell Lung Cancer, PMID: 27856142
Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in epidermal growth factor receptor-2-negative, mesenchymal-epithelial transition-positive gastroesophageal adenocarcinoma: is it a real failure?, PMID: 30363693
Phase I dose-escalation study of onartuzumab as a single agent and in combination with bevacizumab in patients with advanced solid malignancies, PMID: 24493831
HGF as a circulating biomarker of onartuzumab treatment in patients with advanced solid tumors, PMID: 23536720
Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer, PMID: 24101053
A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer, PMID: 28209746
Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer, PMID: 26202594
Phase I study of the anti-MET antibody onartuzumab in patients with solid tumors and MET-positive lung cancer, PMID: 25777467
Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung, PMID: 27937096
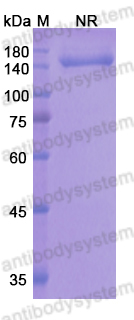
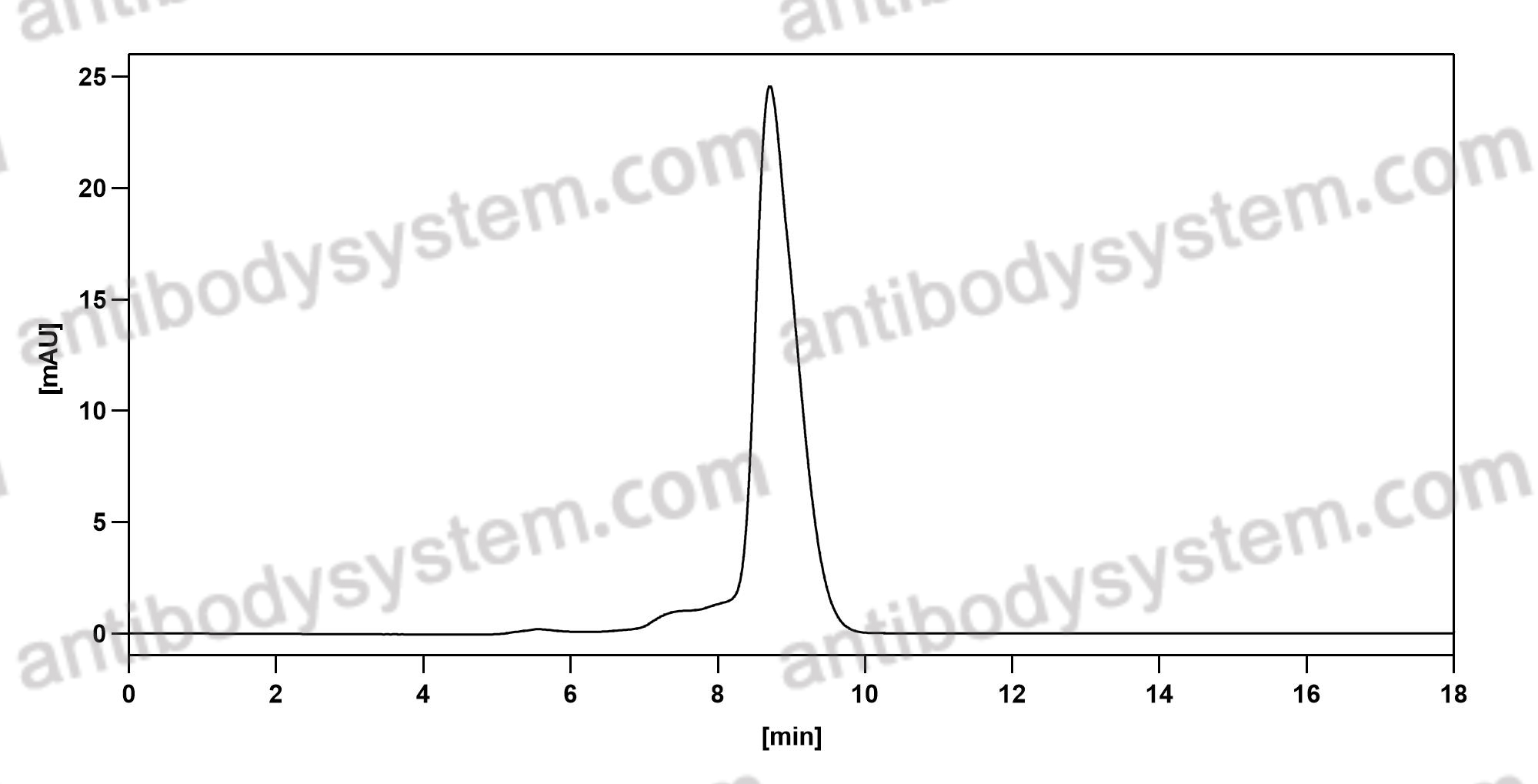
Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent, PMID: 23882082
Exposure-Response and Tumor Growth Inhibition Analyses of the Monovalent Anti-c-MET Antibody Onartuzumab (MetMAb) in the Second- and Third-Line Non-Small Cell Lung Cancer, PMID: 28028730
Nonclinical evaluation of the serum pharmacodynamic biomarkers HGF and shed MET following dosing with the anti-MET monovalent monoclonal antibody onartuzumab, PMID: 24258345
Efficacy and Safety Results From a Phase II, Placebo-Controlled Study of Onartuzumab Plus First-Line Platinum-Doublet Chemotherapy for Advanced Squamous Cell Non-Small-Cell Lung Cancer, PMID: 27461773
Knobs-into-holes antibody production in mammalian cell lines reveals that asymmetric afucosylation is sufficient for full antibody-dependent cellular cytotoxicity, PMID: 23995614
Population pharmacokinetic analysis from phase I and phase II studies of the humanized monovalent antibody, onartuzumab (MetMAb), in patients with advanced solid tumors, PMID: 23922054
Treatment Rationale Study Design for the MetLung Trial: A Randomized, Double-Blind Phase III Study of Onartuzumab (MetMAb) in Combination With Erlotinib Versus Erlotinib Alone in Patients Who Have Received Standard Chemotherapy for Stage IIIB or IV Met-Positive Non-Small-Cell Lung Cancer, PMID: 23063071
PhotoTag: Photoactivatable Fluorophores for Protein Labeling, PMID: 32283028
The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer, PMID: 32575417
Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O 6-Methylguanine-DNA Methyltransferase Biomarker Analyses, PMID: 27918718
ImmunoPET Predicts Response to Met-targeted Radioligand Therapy in Models of Pancreatic Cancer Resistant to Met Kinase Inhibitors, PMID: 31903112
Biomarker development in MET-targeted therapy, PMID: 27013592
Targeting MET and EGFR in NSCLC-what can we learn from the recently reported phase III trial of onartuzumab in combination with erlotinib in advanced non-small cell lung cancer?, PMID: 25806331
Effect of onartuzumab added to erlotinib on metastasis in patients with lung cancer, PMID: 25332242
Treatment rationale and study design for a randomized, double-blind, placebo-controlled phase II study evaluating onartuzumab (MetMAb) in combination with bevacizumab plus mFOLFOX-6 in patients with previously untreated metastatic colorectal cancer, PMID: 23810377
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma, PMID: 30636579
Antibodies to watch in 2014, PMID: 24284914
C-MET inhibitors in the treatment of lung cancer, PMID: 25266653
The emerging role of MET/HGF inhibitors in oncology, PMID: 23453860
Novel agents in development for advanced non-small cell lung cancer, PMID: 25342991
Which are the antibodies to watch in 2013?, PMID: 23254906
Antibodies to watch in 2016, PMID: 26651519
Photoradiosynthesis of 68 Ga-Labeled HBED-CC-Azepin-MetMAb for Immuno-PET of c-MET Receptors, PMID: 31117346
Tracking the 2015 Gastrointestinal Cancers Symposium: bridging cancer biology to clinical gastrointestinal oncology, PMID: 26045669
Immuno-PET Detects Changes in Multi-RTK Tumor Cell Expression Levels in Response to Targeted Kinase Inhibition, PMID: 32646879
Photoradiolabeling of onartuzumab with 99mTc and 188Re-tricarbonyl for radiotheranostics of gastric cancer., PMID:40123688
Erratum: Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine-DNA Methyltransferase Biomarker Analyses., PMID:39388656
Synthesis and Evaluation of a Novel c-Met-Targeting Cyclic Peptide as a Potential Diagnostic Agent for Colorectal Cancer., PMID:38853512
HNODThia: A Promising Chelator for the Development of 64Cu Radiopharmaceuticals., PMID:37487036
Development of a MET-targeted single-chain antibody fragment as an anti-oncogene targeted therapy for breast cancer., PMID:37004643
A rotaxane-based platform for tailoring the pharmacokinetics of cancer-targeted radiotracers., PMID:36519052
Heptadentate chelates for 89Zr-radiolabelling of monoclonal antibodies., PMID:35770072
Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases., PMID:35458666
Efficacy and Safety of Bevacizumab Combined with Other Therapeutic Regimens for Treatment of Recurrent Glioblastoma: A Network Meta-analysis., PMID:34973444
Comparative efficacy and safety of anti-HGF/MET pathway agents plus chemotherapy versus chemotherapy alone as first-line treatment in advanced gastric cancer: a protocol for a systematic review and meta-analysis., PMID:34952869
The Influence of a Polyethylene Glycol Linker on the Metabolism and Pharmacokinetics of a 89Zr-Radiolabeled Antibody., PMID:34056896
Combination of HGF/MET-targeting agents and other therapeutic strategies in cancer., PMID:33497758
The efficacy and safety of onartuzumab in patients with solid cancers: A meta-analysis of randomized trials., PMID:33402588
Immuno-PET Detects Changes in Multi-RTK Tumor Cell Expression Levels in Response to Targeted Kinase Inhibition., PMID:32646879
The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer., PMID:32575417
PhotoTag: Photoactivatable Fluorophores for Protein Labeling., PMID:32283028
Light-Induced Radiosynthesis of 89Zr-DFO-Azepin-Onartuzumab for Imaging the Hepatocyte Growth Factor Receptor., PMID:31924725
ImmunoPET Predicts Response to Met-targeted Radioligand Therapy in Models of Pancreatic Cancer Resistant to Met Kinase Inhibitors., PMID:31903112
Photoradiosynthesis of 68Ga-Labeled HBED-CC-Azepin-MetMAb for Immuno-PET of c-MET Receptors., PMID:31117346
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma., PMID:30636579
First-line onartuzumab plus erlotinib treatment for patients with MET-positive and EGFR mutation-positive non-small-cell lung cancer., PMID:30472556
Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in epidermal growth factor receptor-2-negative, mesenchymal-epithelial transition-positive gastroesophageal adenocarcinoma: is it a real failure?, PMID:30363693
Differential responses of MET activations to MET kinase inhibitor and neutralizing antibody., PMID:30208970
Discovery and Therapeutic Exploitation of Mechanisms of Resistance to MET Inhibitors in Glioblastoma., PMID:30201763
Placental and Fetal Effects of Onartuzumab, a Met/HGF Signaling Antagonist, When Administered to Pregnant Cynomolgus Monkeys., PMID:29893934
Efficacy of osimertinib in a patient with non-small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status., PMID:29399354
Meta-analysis of efficacy and adverse events of erlotinib-based targeted therapies for advanced/metastatic non-small cell lung cancer., PMID:29156837
Does c-Met remain a rational target for therapy in patients with EGFR TKI-resistant non-small cell lung cancer?, PMID:29121501
Is There Merit for MET-Targeted Therapies in Gastroesophageal Cancer?, PMID:28910432
89Zr-Onartuzumab PET imaging of c-MET receptor dynamics., PMID:28315949
Expression of MET in circulating tumor cells correlates with expression in tumor tissue from advanced-stage lung cancer patients., PMID:28212540
A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer., PMID:28209746
Exposure-Response and Tumor Growth Inhibition Analyses of the Monovalent Anti-c-MET Antibody Onartuzumab (MetMAb) in the Second- and Third-Line Non-Small Cell Lung Cancer., PMID:28028730
Onartuzumab ineffective in non-small-cell lung cancer., PMID:28017663
Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung., PMID:27937096
Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial., PMID:27918764
Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase II Study of Onartuzumab Plus Bevacizumab Versus Placebo Plus Bevacizumab in Patients With Recurrent Glioblastoma: Efficacy, Safety, and Hepatocyte Growth Factor and O6-Methylguanine-DNA Methyltransferase Biomarker Analyses., PMID:27918718
Efficacy and Safety of Onartuzumab in Combination With First-Line Bevacizumab- or Pemetrexed-Based Chemotherapy Regimens in Advanced Non-Squamous Non-Small-Cell Lung Cancer., PMID:27856142
Efficacy and Safety Results From a Phase II, Placebo-Controlled Study of Onartuzumab Plus First-Line Platinum-Doublet Chemotherapy for Advanced Squamous Cell Non-Small-Cell Lung Cancer., PMID:27461773
A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction., PMID:27401892
Validation of an electronic program for pathologist training in the interpretation of a complex companion diagnostic immunohistochemical assay., PMID:27349303
Biomarker development in MET-targeted therapy., PMID:27013592
Antibodies to watch in 2016., PMID:26651519
Safety of Onartuzumab in Patients with Solid Tumors: Experience to Date from the Onartuzumab Clinical Trial Program., PMID:26445503
Randomized, phase II, placebo-controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple-negative breast cancer., PMID:26202594
Fibroblasts Influence Survival and Therapeutic Response in a 3D Co-Culture Model., PMID:26053043
Tracking the 2015 Gastrointestinal Cancers Symposium: bridging cancer biology to clinical gastrointestinal oncology., PMID:26045669
Role of mesenchymal-epithelial transition amplification in resistance to anti-epidermal growth factor receptor agents., PMID:25992380
Analysis of KRAS and BRAF genes mutation in the central nervous system metastases of non-small cell lung cancer., PMID:25902737
Onartuzumab in lung cancer: the fall of Icarus?, PMID:25818471