Catalog No.
DHC34201
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
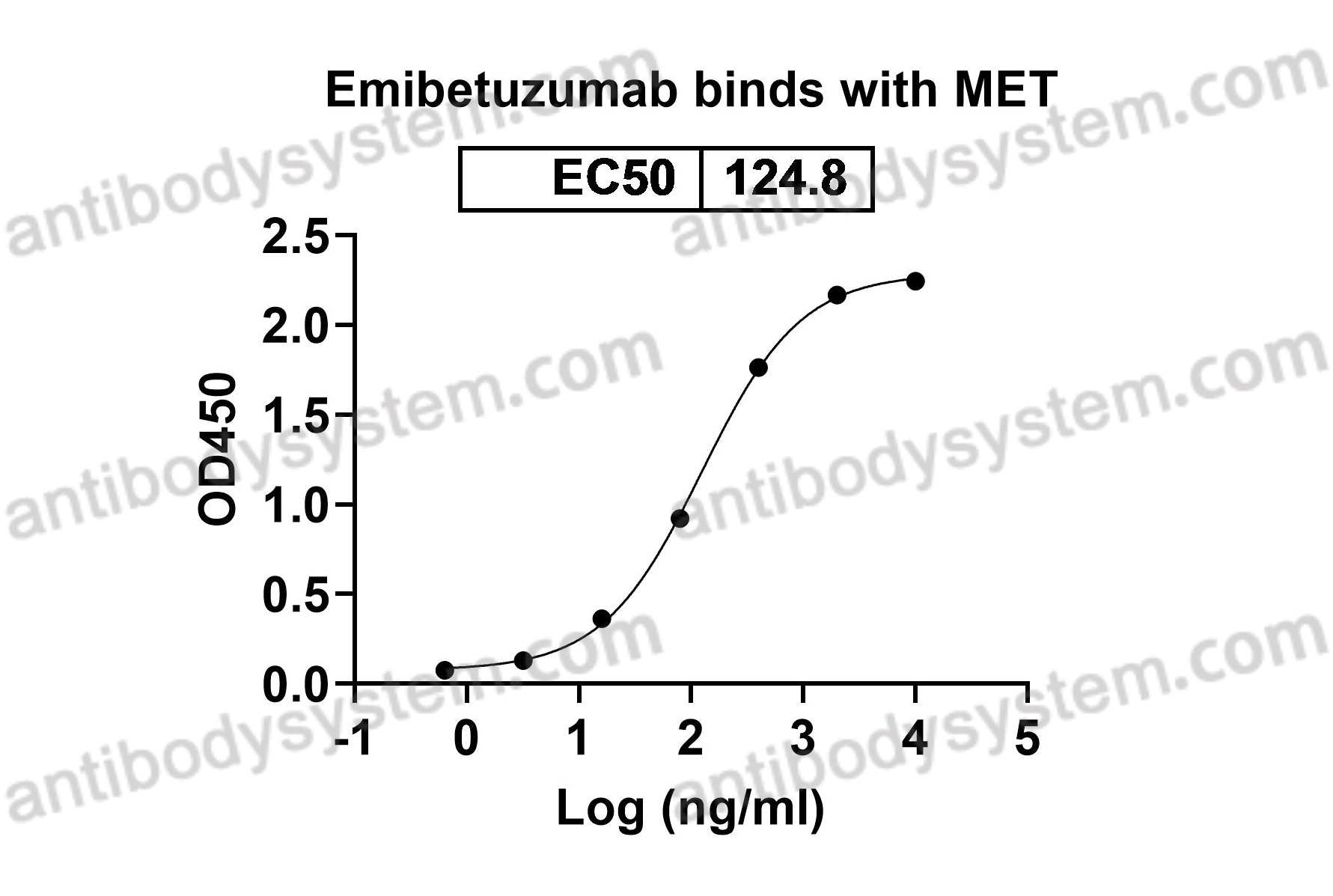
Target
Tyrosine-protein kinase Met, Hepatocyte growth factor receptor, Proto-oncogene c-Met, Scatter factor receptor, HGF receptor, SF receptor, HGF/SF receptor, MET
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
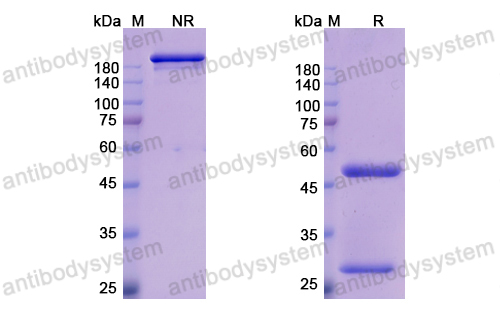
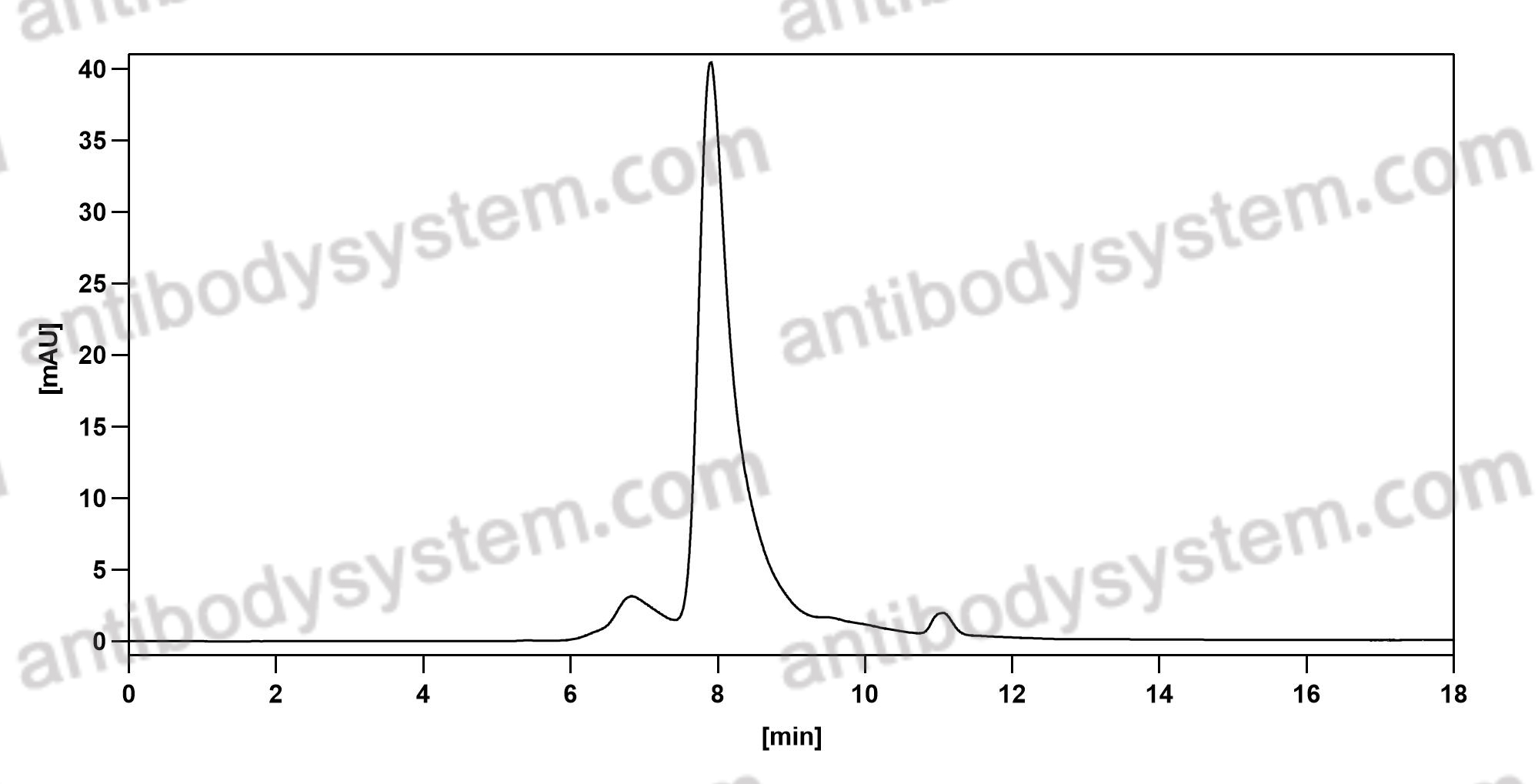
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P08581
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
LA480, LY-2875358, CAS: 1365287-97-3
Clone ID
Emibetuzumab
A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients, PMID: 31622732
A Phase Ib/II Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Cancer, PMID: 31142504
A First-in-Human Phase I Study of a Bivalent MET Antibody, Emibetuzumab (LY2875358), as Monotherapy and in Combination with Erlotinib in Advanced Cancer, PMID: 27803065
MET-targeting antibody (emibetuzumab) and kinase inhibitor (merestinib) as single agent or in combination in a cancer model bearing MET exon 14 skipping, PMID: 29188469
A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer, PMID: 29071414
MARCH Proteins Mediate Responses to Antitumor Antibodies, PMID: 33077644
The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer, PMID: 32575417
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma, PMID: 30636579
Acquired Resistance to a MET Antibody In Vivo Can Be Overcome by the MET Antibody Mixture Sym015, PMID: 29545332
Sym015: A Highly Efficacious Antibody Mixture against MET-Amplified Tumors, PMID: 28679766
Targeted Therapy Approaches for MET Abnormalities in Non-Small Cell Lung Cancer, PMID: 33638808
LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth, PMID: 25231402
A phase I dose-escalation study of LY2875358, a bivalent MET antibody, given as monotherapy or in combination with erlotinib or gefitinib in Japanese patients with advanced malignancies, PMID: 27422720
Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions., PMID:39449330
Evaluating the chaos game representation of proteins for applications in machine learning models: prediction of antibody affinity and specificity as a case study., PMID:37968495
Position-Specific Enrichment Ratio Matrix scores predict antibody variant properties from deep sequencing data., PMID:37503142
Position-Specific Enrichment Ratio Matrix scores predict antibody variant properties from deep sequencing data., PMID:37478351
Co-optimization of therapeutic antibody affinity and specificity using machine learning models that generalize to novel mutational space., PMID:35778381
A Randomized, Open-Label Phase II Study Evaluating Emibetuzumab Plus Erlotinib and Emibetuzumab Monotherapy in MET Immunohistochemistry Positive NSCLC Patients with Acquired Resistance to Erlotinib., PMID:35400584
Targeted Therapy Approaches for MET Abnormalities in Non-Small Cell Lung Cancer., PMID:33638808
MARCH Proteins Mediate Responses to Antitumor Antibodies., PMID:33077644
The Role of MET Inhibitor Therapies in the Treatment of Advanced Non-Small Cell Lung Cancer., PMID:32575417
A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation-Positive NSCLC Patients., PMID:31622732
A Phase Ib/II Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Cancer., PMID:31142504
Targeting the C-MET/HGF Signaling Pathway in Pancreatic Ductal Adenocarcinoma., PMID:30636579
Acquired Resistance to a MET Antibody In Vivo Can Be Overcome by the MET Antibody Mixture Sym015., PMID:29545332
MET-targeting antibody (emibetuzumab) and kinase inhibitor (merestinib) as single agent or in combination in a cancer model bearing MET exon 14 skipping., PMID:29188469
A non-randomized, open-label, single-arm, Phase 2 study of emibetuzumab in Asian patients with MET diagnostic positive, advanced gastric cancer., PMID:29071414
Sym015: A Highly Efficacious Antibody Mixture against MET-Amplified Tumors., PMID:28679766
A First-in-Human Phase I Study of a Bivalent MET Antibody, Emibetuzumab (LY2875358), as Monotherapy and in Combination with Erlotinib in Advanced Cancer., PMID:27803065
A phase I dose-escalation study of LY2875358, a bivalent MET antibody, given as monotherapy or in combination with erlotinib or gefitinib in Japanese patients with advanced malignancies., PMID:27422720
LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth., PMID:25231402