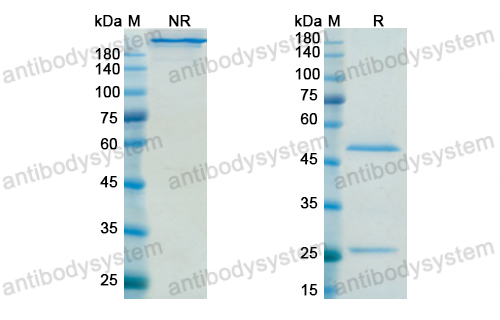
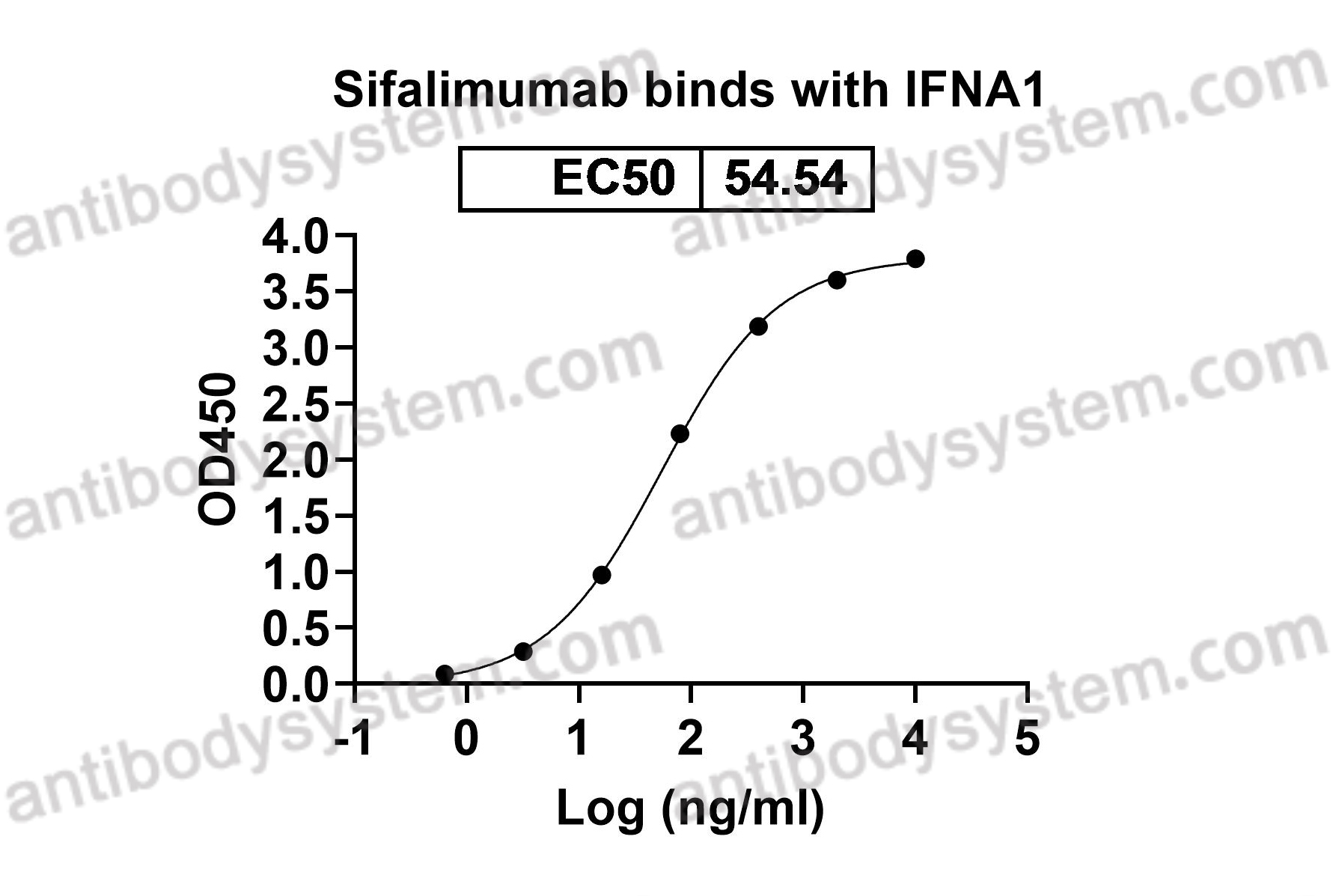
A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients, PMID: 23434567
Additional Improvements in Clinical Response From Adjuvant Biologic Response Modifiers in Adults With Moderate to Severe Systemic Lupus Erythematosus Despite Immunosuppressive Agents: A Systematic Review and Meta-analysis, PMID: 28673504
An Update on the Management of Childhood-Onset Systemic Lupus Erythematosus, PMID: 34244988
Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations, PMID: 32237942
Biologic therapy for autoimmune diseases: an update, PMID: 23557513
Biologic therapy in the idiopathic inflammatory myopathies, PMID: 31680207
Biological therapies and their clinical impact in the treatment of systemic lupus erythematosus, PMID: 31565077
Biologics for idiopathic inflammatory myopathies, PMID: 28817464
Biologics targeting type I interferons in SLE: A meta-analysis and systematic review of randomised controlled trials, PMID: 32960720
Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature, PMID: 21994039
Brain microglia activation induced by intracranial administration of oligonucleotides and its pharmacological modulation, PMID: 29869293
Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus, PMID: 27270345
Efficacy of sifalimumab for treatment of skin injury caused by systemic lupus erythematosus, PMID: 31651869
From mechanism to therapies in systemic lupus erythematosus, PMID: 28118202
Impaired health status and the effect of pain and fatigue on functioning in clinical trial patients with systemic lupus erythematosus, PMID: 24085548
Innate immune-response mechanisms in dermatomyositis: an update on pathogenesis, diagnosis and treatment, PMID: 24939511
Interferon targeted therapies in systemic lupus erythematosus, PMID: 32185249
Interferon α-targeted therapy, PMID: 23994795
Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells?, PMID: 27497254
Modern Therapies for Idiopathic Inflammatory Myopathies (IIMs): Role of Biologics, PMID: 26767526
Population pharmacokinetic analysis of sifalimumab from a clinical phase IIb trial in systemic lupus erythematosus patients, PMID: 26659791
Population pharmacokinetics of sifalimumab, an investigational anti-interferon-α monoclonal antibody, in systemic lupus erythematosus, PMID: 23754736
S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases, PMID: 33748735
Safety and tolerability of sifalimumab, an anti-interferon-α monoclonal antibody, in Japanese patients with systemic lupus erythematosus: A multicenter, phase 2, open-label study, PMID: 30791804
Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study, PMID: 21798883
Scanning for Therapeutic Targets within the Cytokine Network of Idiopathic Inflammatory Myopathies, PMID: 26270565
Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study, PMID: 23400715
Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study, PMID: 27009916
Structural Insights into the Neutralization Properties of the Fully Human, Anti-interferon Monoclonal Antibody Sifalimumab, PMID: 25925951
Suppression of soluble T cell-associated proteins by an anti-interferon-α monoclonal antibody in adult patients with dermatomyositis or polymyositis, PMID: 24357810
Systemic Lupus Erythematosus (SLE) Responder Index response is associated with global benefit for patients with SLE, PMID: 29460699
Systemic Lupus Erythematosus--A Disease with A Dysregulated Type I Interferon System, PMID: 26099519
Targeted therapies in systemic lupus erythematosus, PMID: 23963429
Targeting interferons in systemic lupus erythematosus: current and future prospects, PMID: 25940912
Targeting the interferon pathway with sifalimumab for the treatment of systemic lupus erythematosus, PMID: 28000522
Type I interferon blockade in systemic lupus erythematosus: where do we stand?, PMID: 24344319
Update on clinical trials in systemic lupus erythematosus, PMID: 27314466
Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNα antibody, in systemic lupus erythematosus, PMID: 20392292
Why, why, why de-lupus (does so badly in clinical trials), PMID: 26786849
An Integrative Mechanistic Model of Type 1 IFN-Mediated Inflammation in Systemic Lupus Erythematosus., PMID:40364448
Type I interferon pathway in pediatric systemic lupus erythematosus., PMID:38914753
Model-based meta-analysis using latent variable modeling to set benchmarks for new treatments of systemic lupus erythematosus., PMID:38050332
Safety and efficacy of biological agents in the treatment of Systemic Lupus Erythematosus (SLE)., PMID:37807057
Advances in natural products and antibody drugs for SLE: new therapeutic ideas., PMID:37492083
Current and new targets for treating myositis., PMID:35724455
Antibody Therapies in Autoimmune Inflammatory Myopathies: Promising Treatment Options., PMID:35394612
Inflammatory myopathies: shedding light on promising agents and combination therapies in clinical trials., PMID:34779311
An Update on the Management of Childhood-Onset Systemic Lupus Erythematosus., PMID:34244988
S95021, a novel selective and pan-neutralizing anti interferon alpha (IFN-α) monoclonal antibody as a candidate treatment for selected autoimmune rheumatic diseases., PMID:33748735
Biologics targeting type I interferons in SLE: A meta-analysis and systematic review of randomised controlled trials., PMID:32960720
Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations., PMID:32237942
Biologic therapy in the idiopathic inflammatory myopathies., PMID:31680207
Efficacy of sifalimumab for treatment of skin injury caused by systemic lupus erythematosus., PMID:31651869
Biological therapies and their clinical impact in the treatment of systemic lupus erythematosus., PMID:31565077
Safety and tolerability of sifalimumab, an anti-interferon-α monoclonal antibody, in Japanese patients with systemic lupus erythematosus: A multicenter, phase 2, open-label study., PMID:30791804
Brain microglia activation induced by intracranial administration of oligonucleotides and its pharmacological modulation., PMID:29869293
Systemic Lupus Erythematosus (SLE) Responder Index response is associated with global benefit for patients with SLE., PMID:29460699
Biologics for idiopathic inflammatory myopathies., PMID:28817464
Additional Improvements in Clinical Response From Adjuvant Biologic Response Modifiers in Adults With Moderate to Severe Systemic Lupus Erythematosus Despite Immunosuppressive Agents: A Systematic Review and Meta-analysis., PMID:28673504
Interferon targeted therapies in systemic lupus erythematosus., PMID:32185249
From mechanism to therapies in systemic lupus erythematosus., PMID:28118202
Targeting the interferon pathway with sifalimumab for the treatment of systemic lupus erythematosus., PMID:28000522
Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells?, PMID:27497254
Update on clinical trials in systemic lupus erythematosus., PMID:27314466
Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus., PMID:27270345
Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study., PMID:27009916
Why, why, why de-lupus (does so badly in clinical trials)., PMID:26786849
Modern Therapies for Idiopathic Inflammatory Myopathies (IIMs): Role of Biologics., PMID:26767526
Population pharmacokinetic analysis of sifalimumab from a clinical phase IIb trial in systemic lupus erythematosus patients., PMID:26659791
Scanning for Therapeutic Targets within the Cytokine Network of Idiopathic Inflammatory Myopathies., PMID:26270565
Systemic Lupus Erythematosus--A Disease with A Dysregulated Type I Interferon System., PMID:26099519
Targeting interferons in systemic lupus erythematosus: current and future prospects., PMID:25940912
Structural Insights into the Neutralization Properties of the Fully Human, Anti-interferon Monoclonal Antibody Sifalimumab., PMID:25925951
Innate immune-response mechanisms in dermatomyositis: an update on pathogenesis, diagnosis and treatment., PMID:24939511
Suppression of soluble T cell-associated proteins by an anti-interferon-α monoclonal antibody in adult patients with dermatomyositis or polymyositis., PMID:24357810
Type I interferon blockade in systemic lupus erythematosus: where do we stand?, PMID:24344319
Impaired health status and the effect of pain and fatigue on functioning in clinical trial patients with systemic lupus erythematosus., PMID:24085548
Interferon α-targeted therapy., PMID:23994795
Targeted therapies in systemic lupus erythematosus., PMID:23963429
Population pharmacokinetics of sifalimumab, an investigational anti-interferon-α monoclonal antibody, in systemic lupus erythematosus., PMID:23754736
Biologic therapy for autoimmune diseases: an update., PMID:23557513
A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients., PMID:23434567
Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study., PMID:23400715
Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature., PMID:21994039
Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study., PMID:21798883
Use of type I interferon-inducible mRNAs as pharmacodynamic markers and potential diagnostic markers in trials with sifalimumab, an anti-IFNα antibody, in systemic lupus erythematosus., PMID:20392292