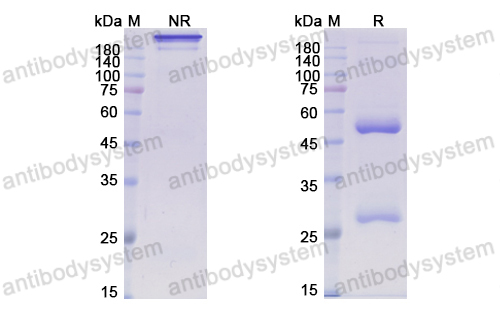
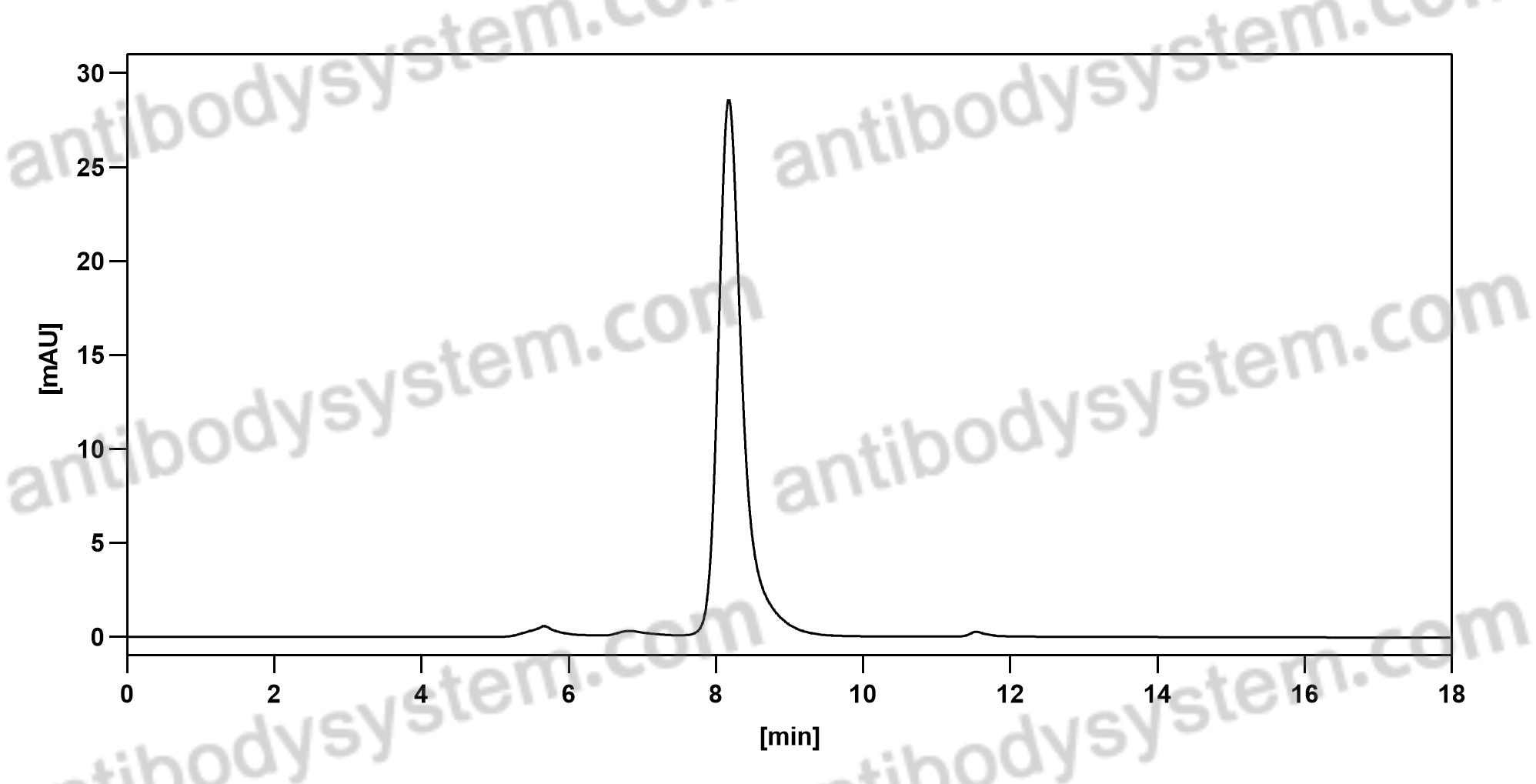
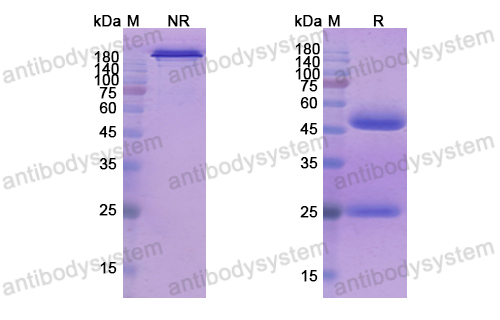
Patritumab or placebo, with cetuximab plus platinum therapy in recurrent or metastatic squamous cell carcinoma of the head and neck: A randomised phase II study, PMID: 31648099
The prospect of patritumab for treating non-small cell lung cancer, PMID: 27744717
Patritumab with Cetuximab plus Platinum-Containing Therapy in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: An Open-Label, Phase Ib Study, PMID: 30327312
Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer, PMID: 30701699
U3-1402, a Novel HER3-Targeting Antibody-Drug Conjugate, for the Treatment of Colorectal Cancer, PMID: 31395690
Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer, PMID: 27452985
A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization, PMID: 31471314
Population pharmacokinetic analysis of patritumab, a HER3 inhibitor, in subjects with advanced non-small cell lung cancer (NSCLC) or solid tumors, PMID: 27017616
Circulating heregulin level is associated with the efficacy of patritumab combined with erlotinib in patients with non-small cell lung cancer, PMID: 28236978
Phase 1 Evaluation of [(64)Cu]DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors, PMID: 26567113
Evaluation of patritumab with or without erlotinib in combination with standard cytotoxic agents against pediatric sarcoma xenograft models, PMID: 29080385
Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors, PMID: 24442032
Phase I study of the HER3-targeted antibody patritumab (U3-1287) combined with erlotinib in Japanese patients with non-small cell lung cancer, PMID: 25891541
The anti-HER3 antibody patritumab abrogates cetuximab resistance mediated by heregulin in colorectal cancer cells, PMID: 25474137
Anti-HER3 monoclonal antibody patritumab sensitizes refractory non-small cell lung cancer to the epidermal growth factor receptor inhibitor erlotinib, PMID: 25961915
Phase 1 study of new formulation of patritumab (U3-1287) Process 2, a fully human anti-HER3 monoclonal antibody in combination with erlotinib in Japanese patients with advanced non-small cell lung cancer, PMID: 28144730
Novel functional anti-HER3 monoclonal antibodies with potent anti-cancer effects on various human epithelial cancers, PMID: 32002122
Clinical Translation and Validation of a Predictive Biomarker for Patritumab, an Anti-human Epidermal Growth Factor Receptor 3 (HER3) Monoclonal Antibody, in Patients With Advanced Non-small Cell Lung Cancer, PMID: 26137564
HER3 and its Ligand, Heregulin, as Targets for Cancer Therapy, PMID: 27086600
SOLTI-1805 TOT-HER3 Study Concept: A Window-of-Opportunity Trial of Patritumab Deruxtecan, a HER3 Directed Antibody Drug Conjugate, in Patients With Early Breast Cancer, PMID: 33968735
Dual targeting of HER3 and MEK may overcome HER3-dependent drug-resistance of colon cancers, PMID: 29312543
HER2-/HER3-Targeting Antibody-Drug Conjugates for Treating Lung and Colorectal Cancers Resistant to EGFR Inhibitors, PMID: 33801379
Real-Time Reverse-Transcription Quantitative Polymerase Chain Reaction Assay Is a Feasible Method for the Relative Quantification of Heregulin Expression in Non-Small Cell Lung Cancer Tissue, PMID: 28469400
Evaluation of Biomarkers for HER3-targeted Therapies in Cancer, PMID: 26137561
Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy, PMID: 25344208
Bayesian adaptive patient enrollment restriction to identify a sensitive subpopulation using a continuous biomarker in a randomized phase 2 trial, PMID: 27485377
Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors, PMID: 23591447
Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma, PMID: 23998444
TUXEDO-3: A phase 2 trial of patritumab deruxtecan for patients with leptomeningeal metastasis., PMID:40461013
Patritumab deruxtecan induces immunogenic cell death., PMID:40458967
Patritumab deruxtecan in leptomeningeal metastatic disease of solid tumors: the phase 2 TUXEDO-3 trial., PMID:40447851
Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials., PMID:40430899
Integrated Two-Analyte Population Pharmacokinetics Model of Patritumab Deruxtecan (HER3-DXd) Monotherapy in Patients with Solid Tumors., PMID:40408047
An evaluation of patritumab deruxtecan for the treatment of EGFR-mutated non-small cell lung cancer., PMID:40374579
Evolving treatment for advanced non-small cell lung cancer harbouring common EGFR activating mutations., PMID:40324662
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Exposure-Response Relationships in Patients with Non-Small-Cell Lung Cancer and Other Solid Tumors Treated with Patritumab Deruxtecan (HER3-DXd)., PMID:40214010
[Standard of care of EGFR mutated metastatic NSCLC in first treatment and beyond progression]., PMID:40155080
Recent advances in therapeutic strategies for non-small cell lung cancer., PMID:40140911
Establishment and characterization of novel patient-derived esophageal tumoroids with long-term cultivability., PMID:40108093
HER3-targeting Antibody-drug Conjugates Therapy for Solid Tumors: Recent Advances and Future Potentials., PMID:39995133
Antibodies to watch in 2025., PMID:39711140
Spotlight on Patritumab Deruxtecan (HER3-DXd) from HERTHENA Lung01. Is a Median PFS of 5.5 Months Enough in Light of FLAURA-2 and MARIPOSA?, PMID:39011085
Patritumab deruxtecan in HER2-negative breast cancer: part B results of the window-of-opportunity SOLTI-1805 TOT-HER3 trial and biological determinants of early response., PMID:38992028
Real-World Treatment Patterns and Clinical Outcomes Among Patients with Metastatic or Unresectable EGFR-Mutated Non-Small Cell Lung Cancer Previously Treated with Osimertinib and Platinum-Based Chemotherapy., PMID:38958845
Long road towards effective HER3 targeting in breast cancer., PMID:38885540
Antibody-drug conjugates in lung and breast cancer: current evidence and future directions-a position statement from the ETOP IBCSG Partners Foundation., PMID:38648979
DB-1310, an ADC comprised of a novel anti-HER3 antibody conjugated to a DNA topoisomerase I inhibitor, is highly effective for the treatment of HER3-positive solid tumors., PMID:38632563
Translational insights and overall survival in the U31402-A-U102 study of patritumab deruxtecan (HER3-DXd) in EGFR-mutated NSCLC., PMID:38369013
Corrigendum: Targeting HER3 to overcome RGFR TKI resistance in NSCLC., PMID:38357544
ERBB3 Overexpression is Enriched in Diverse Patient Populations with Castration-sensitive Prostate Cancer and is Associated with a Unique AR Activity Signature., PMID:38306015
Targeting HER3 to overcome EGFR TKI resistance in NSCLC., PMID:38239350
The Therapeutic Significance of HER3 in Non-small Cell Lung Cancer (NSCLC): A Review Study., PMID:38231075
Antibodies to watch in 2024., PMID:38178784
HERTHENA-Lung02: phase III study of patritumab deruxtecan in advanced EGFR-mutated NSCLC after a third-generation EGFR TKI., PMID:38095056
Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3-Directed Antibody-Drug Conjugate, in Patients With Previously Treated Human Epidermal Growth Factor Receptor 3-Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial., PMID:37801674
Patritumab deruxtecan shows activity in EGFR-mutant NSCLC., PMID:37749383
HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy., PMID:37689979
AMT-562, a Novel HER3-targeting Antibody-Drug Conjugate, Demonstrates a Potential to Broaden Therapeutic Opportunities for HER3-expressing Tumors., PMID:37302522
TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC)., PMID:37230055
HERTHENA-Lung01: a phase II study of patritumab deruxtecan (HER3-DXd) in previously treated metastatic EGFR-mutated NSCLC., PMID:37212796
Patritumab deruxtecan in untreated hormone receptor-positive/HER2-negative early breast cancer: final results from part A of the window-of-opportunity SOLTI TOT-HER3 pre-operative study., PMID:37211044
'Targeting' Improved Outcomes with Antibody-Drug Conjugates in Non-Small Cell Lung Cancer-An Updated Review., PMID:37185443
Functional genomics of human clear cell sarcoma: genomic, transcriptomic and chemical biology landscape for clear cell sarcoma., PMID:36959380
Gain of Aggressive Histological and Molecular Patterns after Acquired Resistance to Novel Anti-EGFR Therapies in Non-Small Cell Lung Cancer., PMID:36835213
Targeting HER3 for cancer treatment: a new horizon for an old target., PMID:36764093
Dual-targeting therapy against HER3/MET in human colorectal cancers., PMID:36751113
Antibody-drug conjugates in lung cancer: dawn of a new era?, PMID:36631624
Quantitative analysis of drug distribution in heterogeneous tissues using dual-stacking capillary electrophoresis-mass spectrometry., PMID:36377519
New Strategies and Novel Combinations in EGFR TKI-Resistant Non-small Cell Lung Cancer., PMID:36242712
Population Pharmacokinetics of Patritumab Deruxtecan in Patients With Solid Tumors., PMID:36053771
Targeting HER3-dependent activation of nuclear AKT improves radiotherapy of non-small cell lung cancer., PMID:35839938
Correction: Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer., PMID:35652222
Patritumab deruxtecan (HER3-DXd), a novel HER3 directed antibody drug conjugate, exhibits in vitro activity against breast cancer cells expressing HER3 mutations with and without HER2 overexpression., PMID:35503762
Patritumab Deruxtecan: Paving the Way for EGFR-TKI-Resistant NSCLC., PMID:35022206
Antibody-Drug Conjugates: A New Addition to the Treatment Landscape of EGFR-Mutant Non-Small Cell Lung Cancer., PMID:34983785
HER3 Augmentation via Blockade of EGFR/AKT Signaling Enhances Anticancer Activity of HER3-Targeting Patritumab Deruxtecan in EGFR-Mutated Non-Small Cell Lung Cancer., PMID:34921025
HER3 Is an Actionable Target in Advanced Prostate Cancer., PMID:34753775