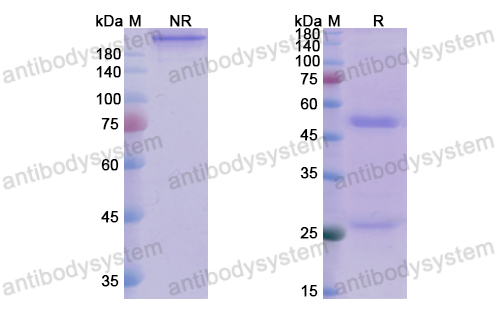
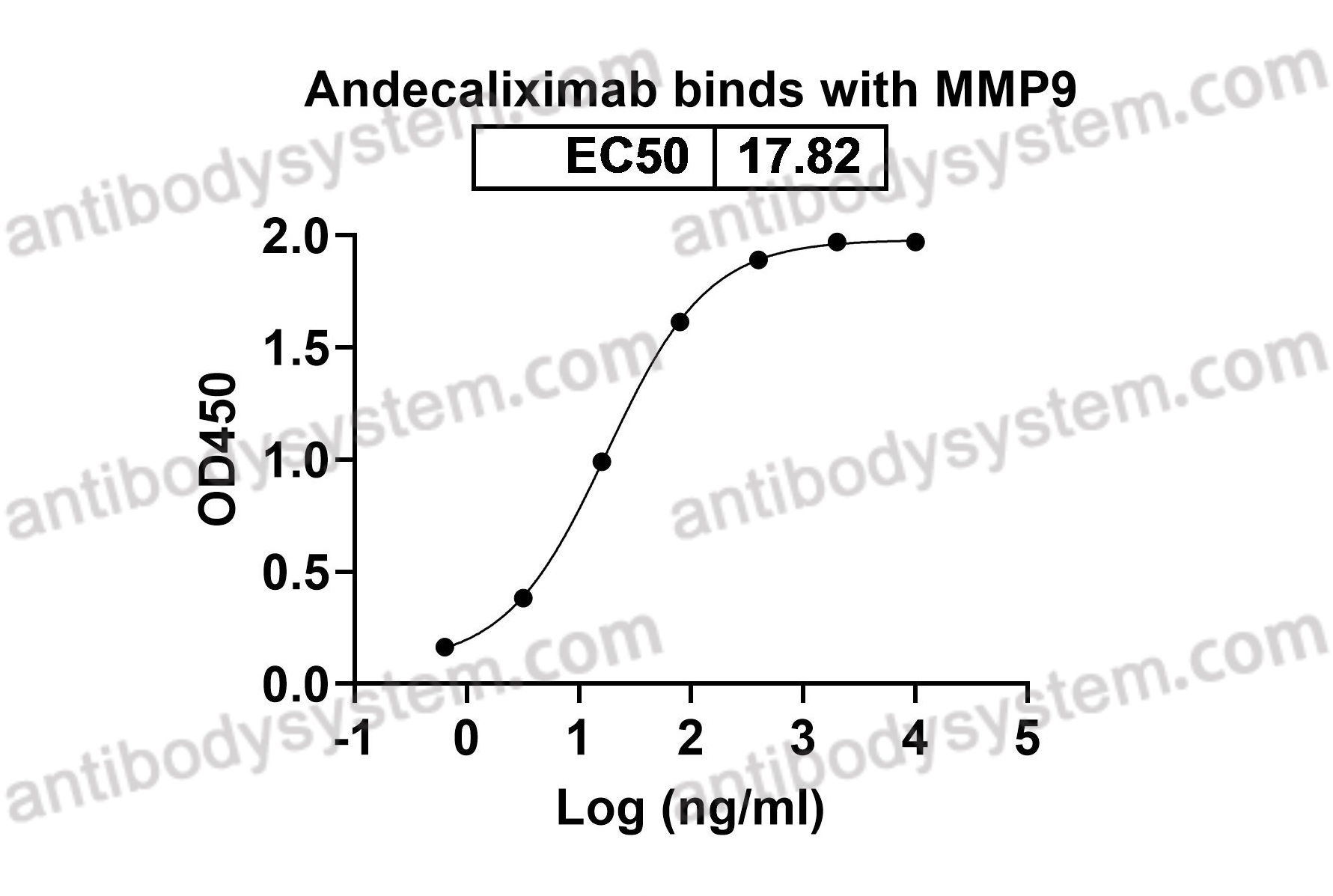
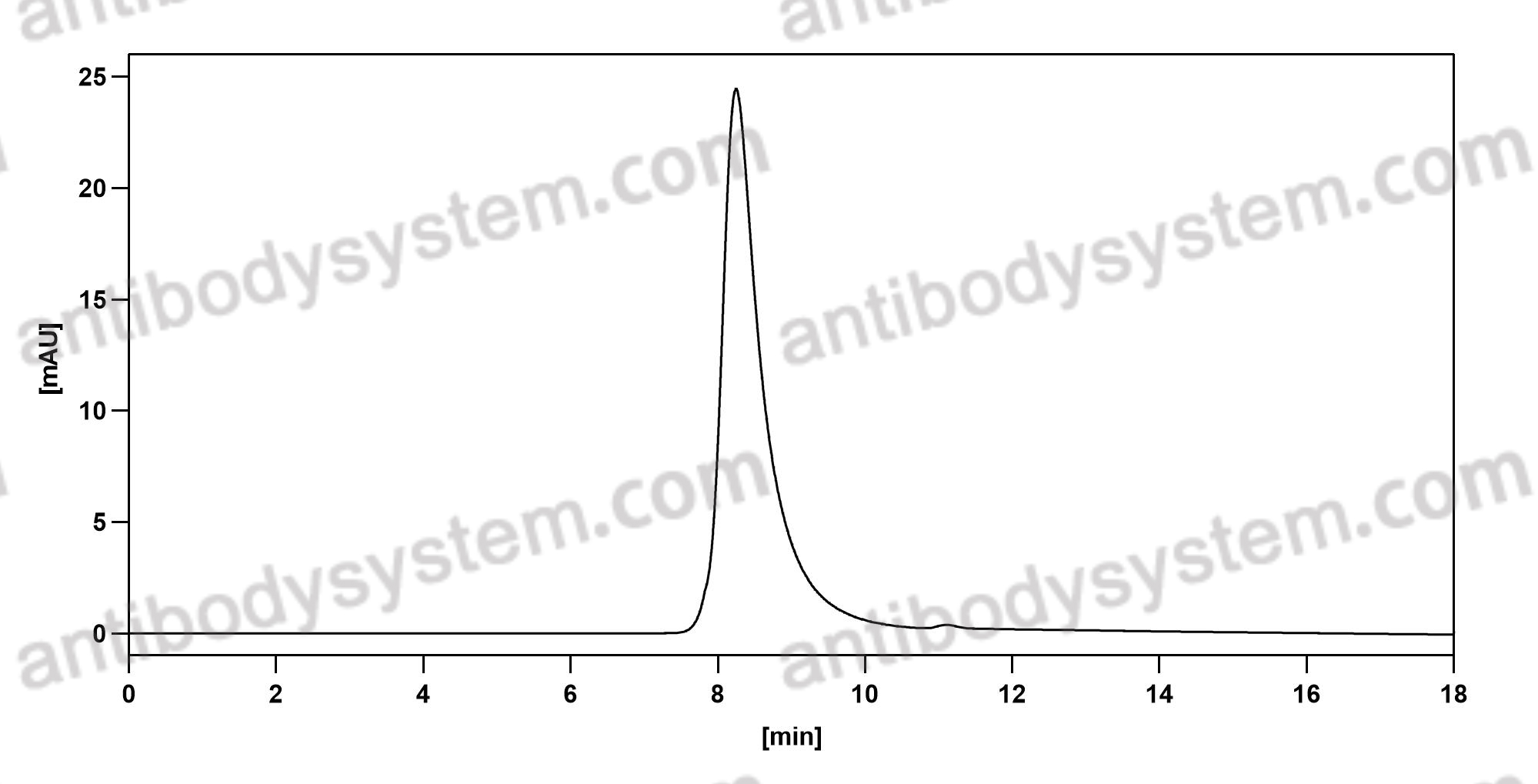
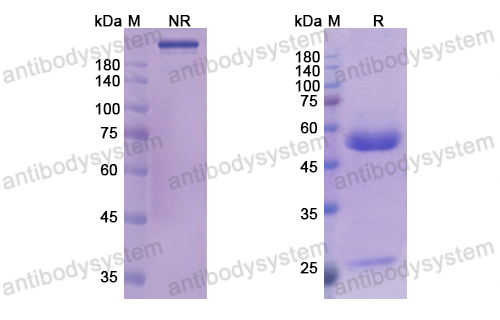
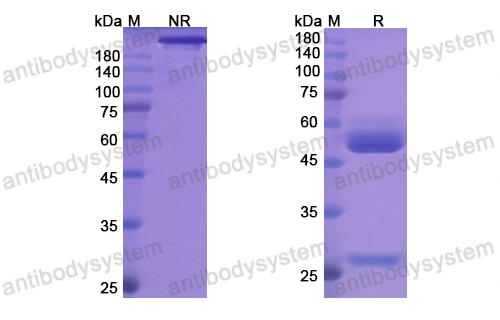
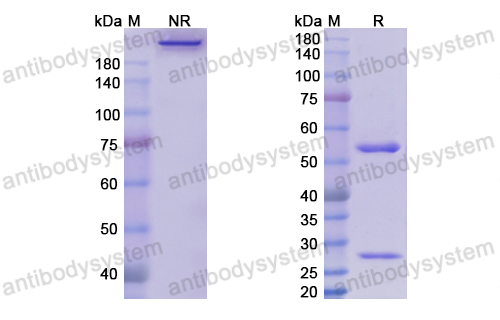
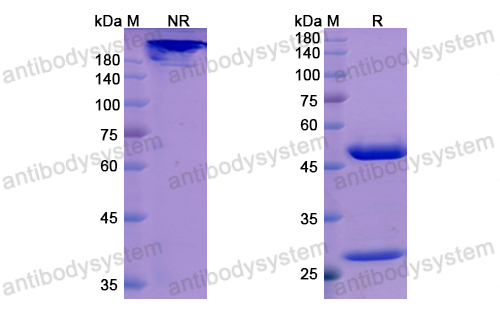
Catalog No.
DHD06801
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Chimeric
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
Matrix metalloproteinase-9, CLG4B, GELB, 92 kDa type IV collagenase, 92 kDa gelatinase, Gelatinase B, MMP9, MMP-9
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P14780
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
GS-5745, CAS: 1518996-49-0
Clone ID
Andecaliximab
Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study, PMID: 29691300
Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease, PMID: 29767728
Safety and Efficacy of Andecaliximab (GS-5745) Plus Gemcitabine and Nab-Paclitaxel in Patients with Advanced Pancreatic Adenocarcinoma: Results from a Phase I Study, PMID: 32812320
A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn's Disease, PMID: 29846530
Phase 1b Study of the Safety, Pharmacokinetics, and Disease-related Outcomes of the Matrix Metalloproteinase-9 Inhibitor Andecaliximab in Patients With Rheumatoid Arthritis, PMID: 29287749
Phase III Study to Evaluate Efficacy and Safety of Andecaliximab With mFOLFOX6 as First-Line Treatment in Patients With Advanced Gastric or GEJ Adenocarcinoma (GAMMA-1), PMID: 33577358
Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis, PMID: 33052708
Emerging Therapies in the Management of Advanced-Stage Gastric Cancer, PMID: 30271341
Matrix metalloproteinases in the pathogenesis of dengue viral disease: Involvement of immune system and newer therapeutic strategies, PMID: 33634515
Letter: anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 for ulcerative colitis, PMID: 27593425
Failure of MMP-9 Antagonists in IBD: Demonstrating the Importance of Molecular Biology and Well-Controlled Early Phase Studies, PMID: 30010739
Randomised clinical trial: a phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis, PMID: 27218676
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Inside a Metastatic Fracture: Molecular Bases and New Potential Therapeutic Targets., PMID:40304052
Text Mining and Drug Discovery Analysis: A Comprehensive Approach to Investigate Diabetes-Induced Osteoporosis., PMID:38250601
Identification of key genes in colorectal cancer diagnosis by co-expression analysis weighted gene co-expression network analysis., PMID:36931200
Characterization of Active MMP9 in Chronic Inflammatory Diseases Using a Novel Anti-MMP9 Antibody., PMID:36810514
Evaluation of the matrix metalloproteinase 9 (MMP9) inhibitor Andecaliximab as an Anti-invasive therapeutic in Head and neck squamous cell carcinoma., PMID:35803110
A phase 1b study of andecaliximab in combination with S-1 plus platinum in Japanese patients with gastric adenocarcinoma., PMID:35773363
Safety and tolerability of andecaliximab as monotherapy and in combination with an anti-PD-1 antibody in Japanese patients with gastric or gastroesophageal junction adenocarcinoma: a phase 1b study., PMID:34992093
Randomized, open-label, phase 2 study of andecaliximab plus nivolumab versus nivolumab alone in advanced gastric cancer identifies biomarkers associated with survival., PMID:34893523
Matrix metalloproteinases in the pathogenesis of dengue viral disease: Involvement of immune system and newer therapeutic strategies., PMID:33634515
Phase III Study to Evaluate Efficacy and Safety of Andecaliximab With mFOLFOX6 as First-Line Treatment in Patients With Advanced Gastric or GEJ Adenocarcinoma (GAMMA-1)., PMID:33577358
Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis., PMID:33052708
Safety and Efficacy of Andecaliximab (GS-5745) Plus Gemcitabine and Nab-Paclitaxel in Patients with Advanced Pancreatic Adenocarcinoma: Results from a Phase I Study., PMID:32812320
Emerging Therapies in the Management of Advanced-Stage Gastric Cancer., PMID:30271341
Failure of MMP-9 Antagonists in IBD: Demonstrating the Importance of Molecular Biology and Well-Controlled Early Phase Studies., PMID:30010739
A Phase 2, Randomized, Placebo-Controlled Study Evaluating Matrix Metalloproteinase-9 Inhibitor, Andecaliximab, in Patients With Moderately to Severely Active Crohn's Disease., PMID:29846530
Andecaliximab [Anti-matrix Metalloproteinase-9] Induction Therapy for Ulcerative Colitis: A Randomised, Double-Blind, Placebo-Controlled, Phase 2/3 Study in Patients With Moderate to Severe Disease., PMID:29767728
Andecaliximab/GS-5745 Alone and Combined with mFOLFOX6 in Advanced Gastric and Gastroesophageal Junction Adenocarcinoma: Results from a Phase I Study., PMID:29691300
Phase 1b Study of the Safety, Pharmacokinetics, and Disease-related Outcomes of the Matrix Metalloproteinase-9 Inhibitor Andecaliximab in Patients With Rheumatoid Arthritis., PMID:29287749
Letter: anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 for ulcerative colitis., PMID:27593425
Randomised clinical trial: a phase 1, dose-ranging study of the anti-matrix metalloproteinase-9 monoclonal antibody GS-5745 versus placebo for ulcerative colitis., PMID:27218676