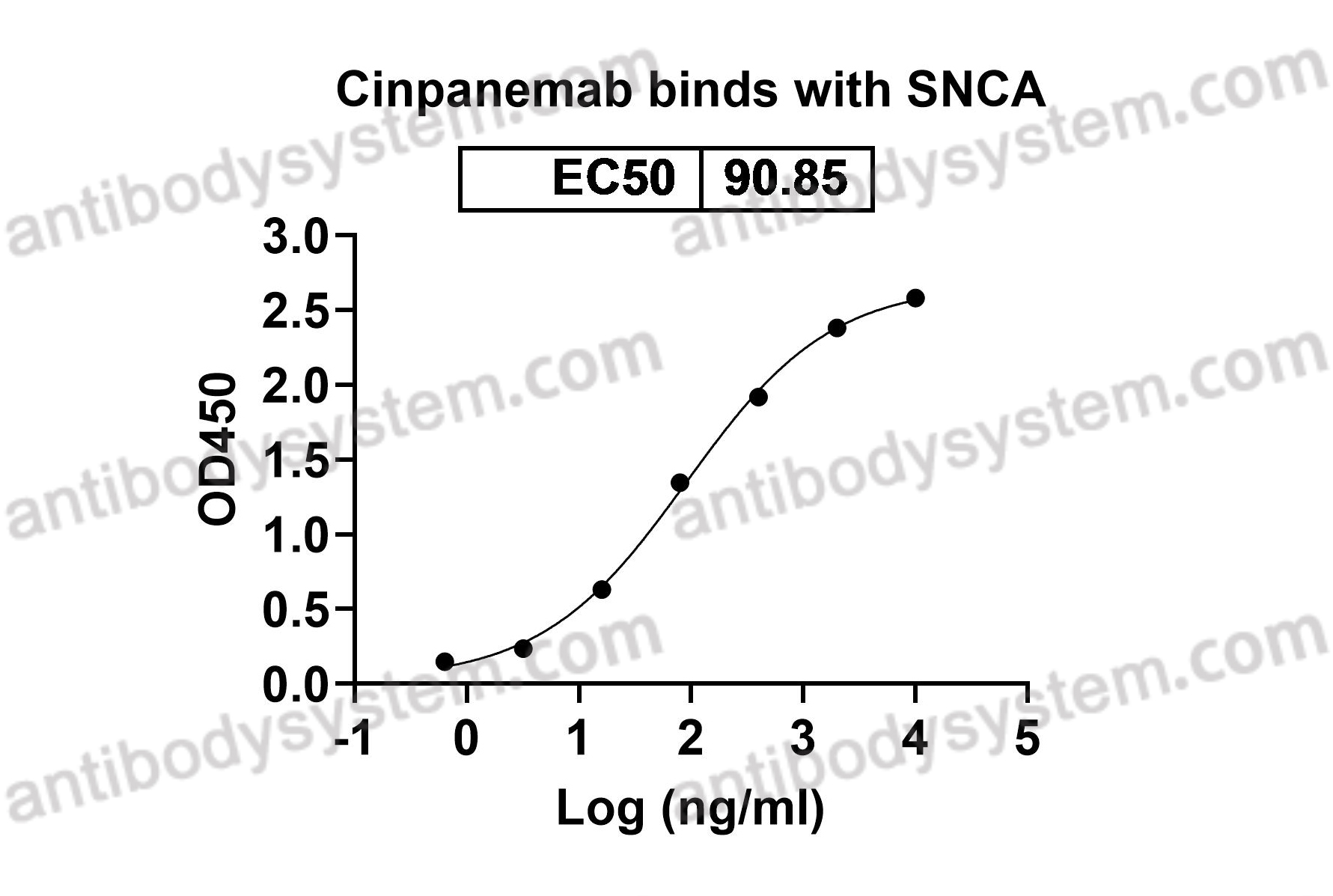
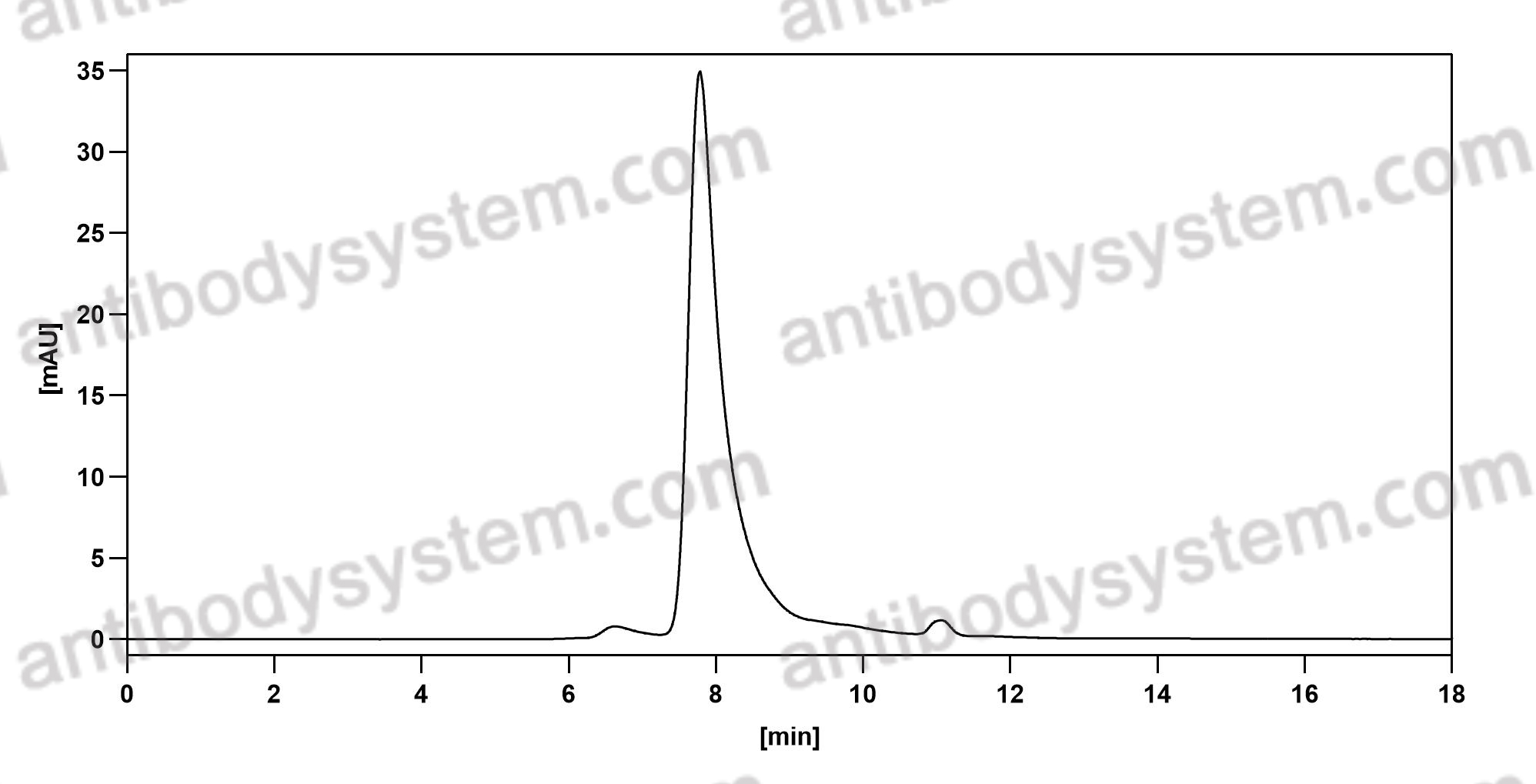
Catalog No.
DHE22001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-lambda
Clonality
Monoclonal
Target
Alpha-synuclein, PARK1, Non-A4 component of amyloid precursor, NACP, Non-A beta component of AD amyloid, SNCA
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P37840
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BIIB054,BIIB-054, CAS: 2094516-02-4
Clone ID
Cinpanemab
Phase II Dose Selection for Alpha Synuclein-Targeting Antibody Cinpanemab (BIIB054) Based on Target Protein Binding Levels in the Brain, PMID: 32613752
Immunotherapies for Parkinson's disease: Progression of Clinical Development, PMID: 34042040
Facile generation of drug-like conformational antibodies specific for amyloid fibrils., PMID:40301692
Quantification of cinpanemab (BIIB054) binding to α-synuclein in cerebrospinal fluid of phase 1 single ascending dose samples., PMID:39892989
An update on immune-based alpha-synuclein trials in Parkinson's disease., PMID:39666171
Limitations and potential strategies of immune checkpoint blockade in age-related neurodegenerative disorders., PMID:39313800
Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders., PMID:38928395
Transcytosis-Driven Treatment of Neurodegenerative Disorders by mRNA-Expressed Antibody-Transferrin Conjugates., PMID:38672205
Cinpanemab in Early Parkinson Disease: Evaluation of Biomarker Results From the Phase 2 SPARK Clinical Trial., PMID:38315945
The Construction and Validation of a Novel Ferroptosis-Related Gene Signature in Parkinson's Disease., PMID:38139032
Single-neuron neurodegeneration as a degenerative model for Parkinson's disease., PMID:37721280
Analysis of clinical failure of anti-tau and anti-synuclein antibodies in neurodegeneration using a quantitative systems pharmacology model., PMID:37658103
Flow cytometric isolation of drug-like conformational antibodies specific for amyloid fibrils., PMID:37461643
Monoclonal Antibodies in Neurodegenerative Disease May Work, But They Don't Help: A Perspective from Physicians., PMID:36442210
Prasinezumab and Cinpanemab - The Perspective of a Person with Parkinson's., PMID:36373296
Trial of Cinpanemab in Early Parkinson's Disease., PMID:35921450
Evaluating dopamine transporter imaging as an enrichment biomarker in a phase 2 Parkinson's disease trial., PMID:34814867
Immunotherapies for Parkinson's Disease: Progression of Clinical Development., PMID:34042040
Phase II Dose Selection for Alpha Synuclein-Targeting Antibody Cinpanemab (BIIB054) Based on Target Protein Binding Levels in the Brain., PMID:32613752