Catalog No.
DHC82404
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
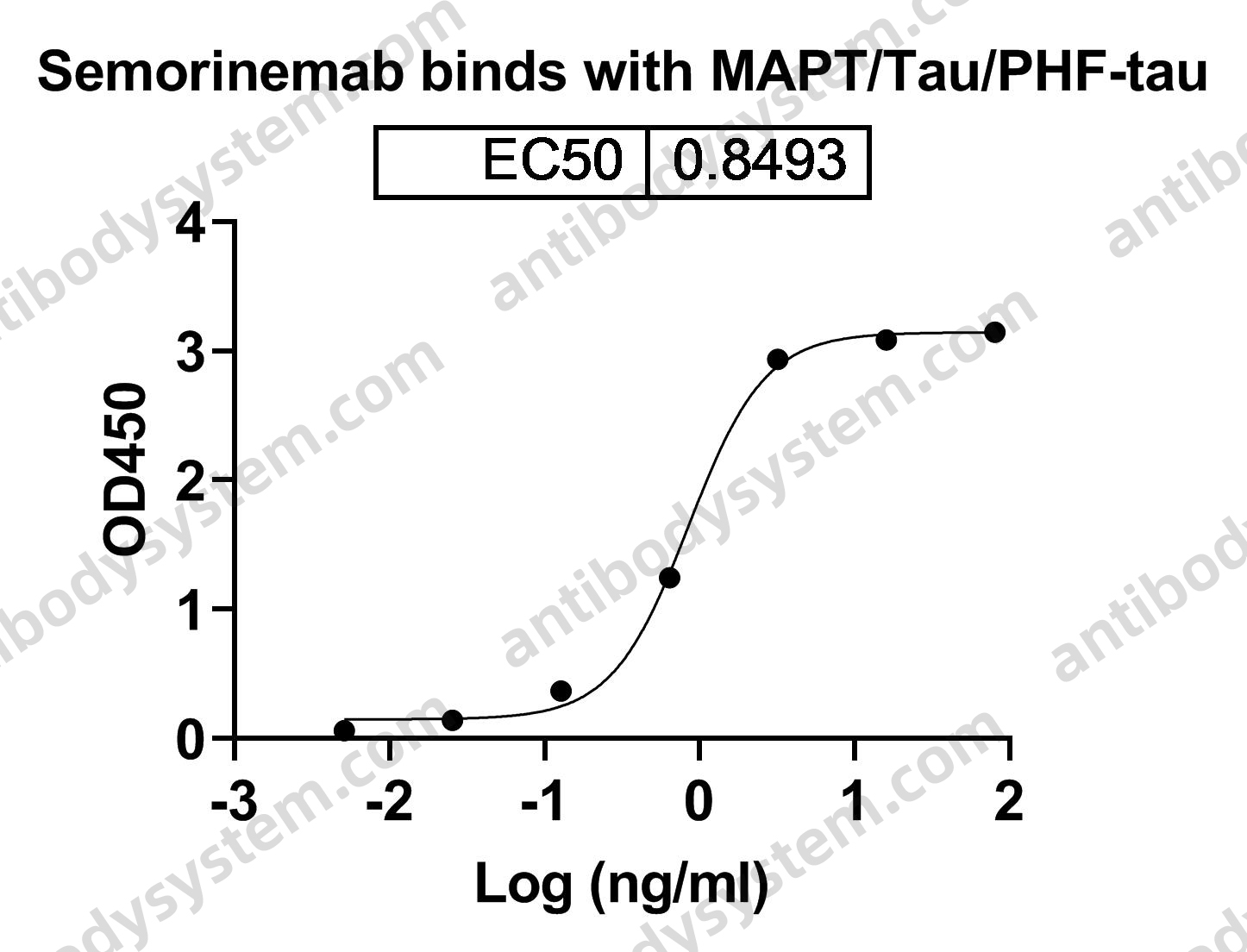
MAPTL, Paired helical filament-tau, MTBT1, Microtubule-associated protein tau, TAU, Neurofibrillary tangle protein, MAPT, PHF-tau
Concentration
2.49 mg/ml
Endotoxin level
Please contact with the lab for this information.
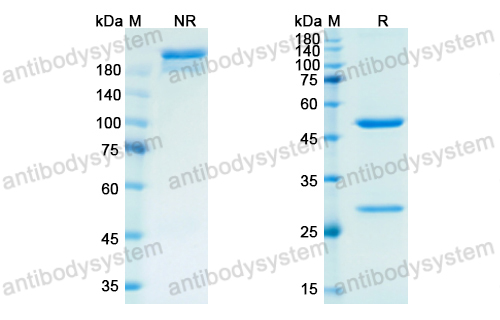
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P10636
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
RO-7105705, CAS: 2159141-27-0
Clone ID
Semorinemab
Alzheimer's disease: Recent treatment strategies, PMID: 32941929
Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer's disease, PMID: 33980574
CSF proteomics of semorinemab Alzheimer's disease trials identifies cell-type specific signatures., PMID:40435316
The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer's disease based solely on biomarker evidence., PMID:40243238
Lecanemab for early Alzheimer's disease: Appropriate use recommendations from the French federation of memory clinics., PMID:40011173
Comparative the efficacy and safety of Gosuranemab, Semorinemab, Tilavonemab, and Zagotenemab in patients with Alzheimer's disease: a systematic review and network meta-analysis of randomized controlled trials., PMID:39945003
Phase 2A Proof-of-Concept Double-Blind, Randomized, Placebo-Controlled Trial of Nicotinamide in Early Alzheimer Disease., PMID:39671543
Pharmacodynamic effects of semorinemab on plasma and CSF biomarkers of Alzheimer's disease pathophysiology., PMID:39513754
2024 AA criteria for Alzheimer's disease diagnosis: Mainly anchored at Aβ not tau., PMID:39470319
CSF complement proteins are elevated in prodromal to moderate Alzheimer's disease patients and are not altered by the anti-tau antibody semorinemab., PMID:39369294
Semorinemab Pharmacokinetics and The Effect on Plasma Total Tau Pharmacodynamics in Clinical Studies., PMID:39350369
Scenarios for the long-term efficacy of amyloid-targeting therapies in the context of the natural history of Alzheimer's disease., PMID:39073291
A Review of Recent Advances in the Management of Alzheimer's Disease., PMID:38756263
What is Alzheimer's disease? An analysis of nosological perspectives from the 20th and 21st centuries., PMID:38618742
Evaluation of partial volume correction and analysis of longitudinal [18F]GTP1 tau PET imaging in Alzheimer's disease using linear mixed-effects models., PMID:38606196
Prognostic and Predictive Factors in Early Alzheimer's Disease: A Systematic Review., PMID:38405341
Posterior cortical atrophy: new insights into treatments and biomarkers for Alzheimer's disease., PMID:38267172
Analysis of clinical failure of anti-tau and anti-synuclein antibodies in neurodegeneration using a quantitative systems pharmacology model., PMID:37658103
Randomized Phase II Study of the Safety and Efficacy of Semorinemab in Participants With Mild-to-Moderate Alzheimer Disease: Lauriet., PMID:37643887
Semorinemab in Mild-to-Moderate Alzheimer Disease: A Glimmer of Hope Though Cautions Remain., PMID:37643886
Passive tau-based immunotherapy for tauopathies., PMID:37620094
Clinical development of passive tau-based immunotherapeutics for treating primary and secondary tauopathies., PMID:37405389
Key questions for the evaluation of anti-amyloid immunotherapies for Alzheimer's disease., PMID:37389302
Cross-sectional and longitudinal assessments of function in prodromal-to-mild Alzheimer's disease: A comparison of the ADCS-ADL and A-IADL-Q scales., PMID:37325545
Dementia prevention in memory clinics: recommendations from the European task force for brain health services., PMID:36895446
The Use of Episodic Memory Tests for Screening in Clinical Trials for Early Alzheimer's Disease: A Comparison of the Free and Cued Selective Reminding Test (FCSRT) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)., PMID:36641609
[Therapeutic news in Alzheimer’s disease: soon a disease-modifying therapy?]., PMID:35929392
Anti-Tau Antibody Semorinemab Fails to Slow Alzheimer Disease., PMID:35916858
Safety and Efficacy of Semorinemab in Individuals With Prodromal to Mild Alzheimer Disease: A Randomized Clinical Trial., PMID:35696185
Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer's disease., PMID:33980574
Alzheimer's disease: Recent treatment strategies., PMID:32941929