Catalog No.
DHK08301
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG2-kappa
Clonality
Monoclonal
Target
AICD-57, A4, Beta-CTF, Protease nexin-II, Gamma-CTF(50), ABPP, S-APP-beta, APP, Abeta40, Abeta42, AID(59), Amyloid intracellular domain 59, AID(57), S-APP-alpha, Gamma-CTF(59), Beta-secretase C-terminal fragment, Amyloid-beta precursor protein, Amyloid precursor protein, Beta-APP42, PreA4, Alzheimer disease amyloid protein, APPI, PN-II, AICD-59, Alpha-secretase C-terminal fragment, Amyloid-beta A4 protein, Amyloid intracellular domain 57, CVAP, Amyloid intracellular domain 50, Beta-APP40, AD1, Cerebral vascular amyloid peptide, AID(50), Alpha-CTF, Gamma-CTF(57), AICD-50
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
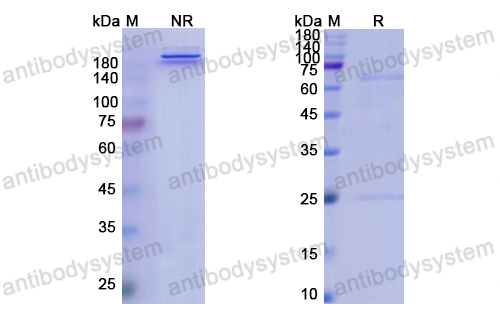
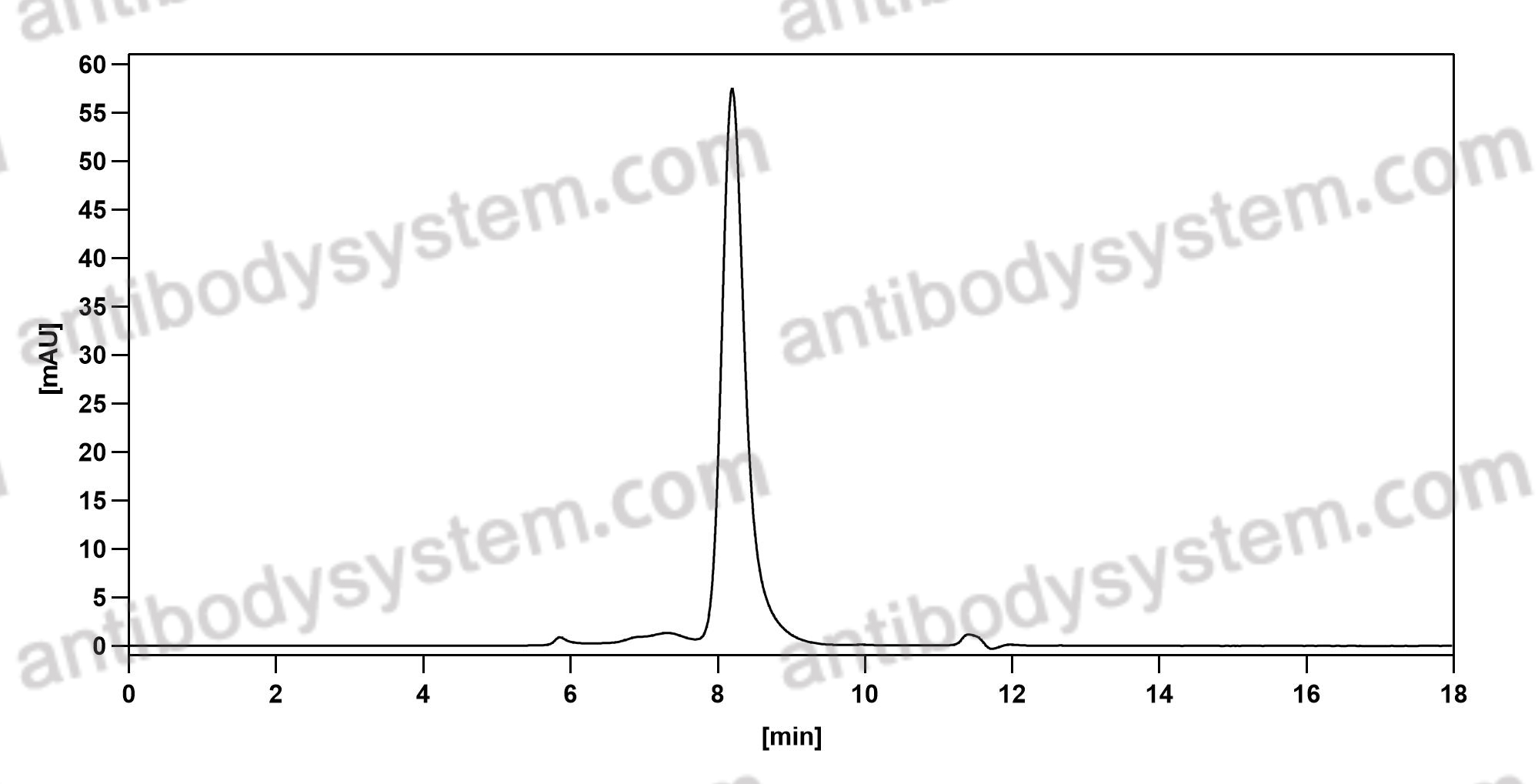
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P05067
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
PF-04360365, RN-1219, clone 9TL, CAS: 1178862-65-1
Clone ID
Ponezumab
Immunotherapy with ponezumab for probable cerebral amyloid angiopathy, PMID: 31020004
Ponezumab in mild-to-moderate Alzheimer's disease: Randomized phase II PET-PIB study, PMID: 29067345
Multiple-dose ponezumab for mild-to-moderate Alzheimer's disease: Safety and efficacy, PMID: 29067341
The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice, PMID: 28844070
39-week toxicity and toxicokinetic study of ponezumab (PF-04360365) in cynomolgus monkeys with 12-week recovery period, PMID: 22045481
Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer's disease, PMID: 22197375
Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild to moderate Alzheimer disease, PMID: 23334069
Passive immunotherapy targeting amyloid-β reduces cerebral amyloid angiopathy and improves vascular reactivity, PMID: 26493635
Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study, PMID: 23334070
Chronic administration of an aglycosylated murine antibody of ponezumab does not worsen microhemorrhages in aged Tg2576 mice, PMID: 22631613
Clearance of amyloid-beta in Alzheimer's disease: shifting the action site from center to periphery, PMID: 24733588
Recent Updates in the Alzheimer's Disease Etiopathology and Possible Treatment Approaches: A Narrative Review of Current Clinical Trials, PMID: 32321414
Structural aspects of Alzheimer's disease immunotherapy targeted against amyloid-beta peptide, PMID: 29663816
Thousands of amyloids may foil Alzheimer's drugs, PMID: 30718876
[Antibody therapy for Alzheimer's disease], PMID: 22277519
Off-label use of aducanumab for cerebral amyloid angiopathy, PMID: 34237272
Safety and pharmacokinetics of PF-04360365 following a single-dose intravenous infusion in Japanese subjects with mild-to-moderate Alzheimer's disease: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation study, PMID: 24131736
Development of amyloid beta-directed antibodies against Alzheimer's disease: Twists and turns., PMID:38220210
Off-label use of aducanumab for cerebral amyloid angiopathy., PMID:34237272
Passive immunotherapies targeting Aβ and tau in Alzheimer's disease., PMID:32682954
Recent Updates in the Alzheimer's Disease Etiopathology and Possible Treatment Approaches: A Narrative Review of Current Clinical Trials., PMID:32321414
Immunotherapy with ponezumab for probable cerebral amyloid angiopathy., PMID:31020004
Thousands of amyloids may foil Alzheimer's drugs., PMID:30718876
Structural aspects of Alzheimer's disease immunotherapy targeted against amyloid-beta peptide., PMID:29663816
Ponezumab in mild-to-moderate Alzheimer's disease: Randomized phase II PET-PIB study., PMID:29067345
Multiple-dose ponezumab for mild-to-moderate Alzheimer's disease: Safety and efficacy., PMID:29067341
The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice., PMID:28844070
Passive immunotherapy targeting amyloid-β reduces cerebral amyloid angiopathy and improves vascular reactivity., PMID:26493635
Clearance of amyloid-beta in Alzheimer's disease: shifting the action site from center to periphery., PMID:24733588
Safety and pharmacokinetics of PF-04360365 following a single-dose intravenous infusion in Japanese subjects with mild-to-moderate Alzheimer's disease: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation study., PMID:24131736
Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study., PMID:23334070
Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild to moderate Alzheimer disease., PMID:23334069
Chronic administration of an aglycosylated murine antibody of ponezumab does not worsen microhemorrhages in aged Tg2576 mice., PMID:22631613
[Antibody therapy for Alzheimer's disease]., PMID:22277519
Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer's disease., PMID:22197375
39-week toxicity and toxicokinetic study of ponezumab (PF-04360365) in cynomolgus monkeys with 12-week recovery period., PMID:22045481