Catalog No.
DHC12504
Description
Aducanumab (brand name: Aduhelm) is an amyloid beta-directed monoclonal antibody that targets amyloid beta in the brains of people with Alzheimer’s to reduce its buildup. Aducanumab is a monoclonal antibody (a protein that helps your immune system target other proteins), and it is designed to help your body remove something called amyloid beta from the brain. Amyloid beta is an important protein involved in the progression of Alzheimer’s disease. Aducanumab is given intravenously (infused through a vein) monthly. Aducanumab does not cure Alzheimer’s disease, but one (of two) large clinical studies suggests that aducanumab may slow the progression of the earliest symptoms of Alzheimer’s disease.
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
AICD-57, A4, Beta-CTF, Protease nexin-II, Gamma-CTF(50), ABPP, S-APP-beta, APP, Abeta40, Abeta42, AID(59), Amyloid intracellular domain 59, AID(57), S-APP-alpha, Gamma-CTF(59), Beta-secretase C-terminal fragment, Amyloid-beta precursor protein, Amyloid precursor protein, Beta-APP42, PreA4, Alzheimer disease amyloid protein, APPI, PN-II, AICD-59, Alpha-secretase C-terminal fragment, Amyloid-beta A4 protein, Amyloid intracellular domain 57, CVAP, Amyloid intracellular domain 50, Beta-APP40, AD1, Cerebral vascular amyloid peptide, AID(50), Alpha-CTF, Gamma-CTF(57), AICD-50
Concentration
2.3 mg/ml
Endotoxin level
Please contact with the lab for this information.
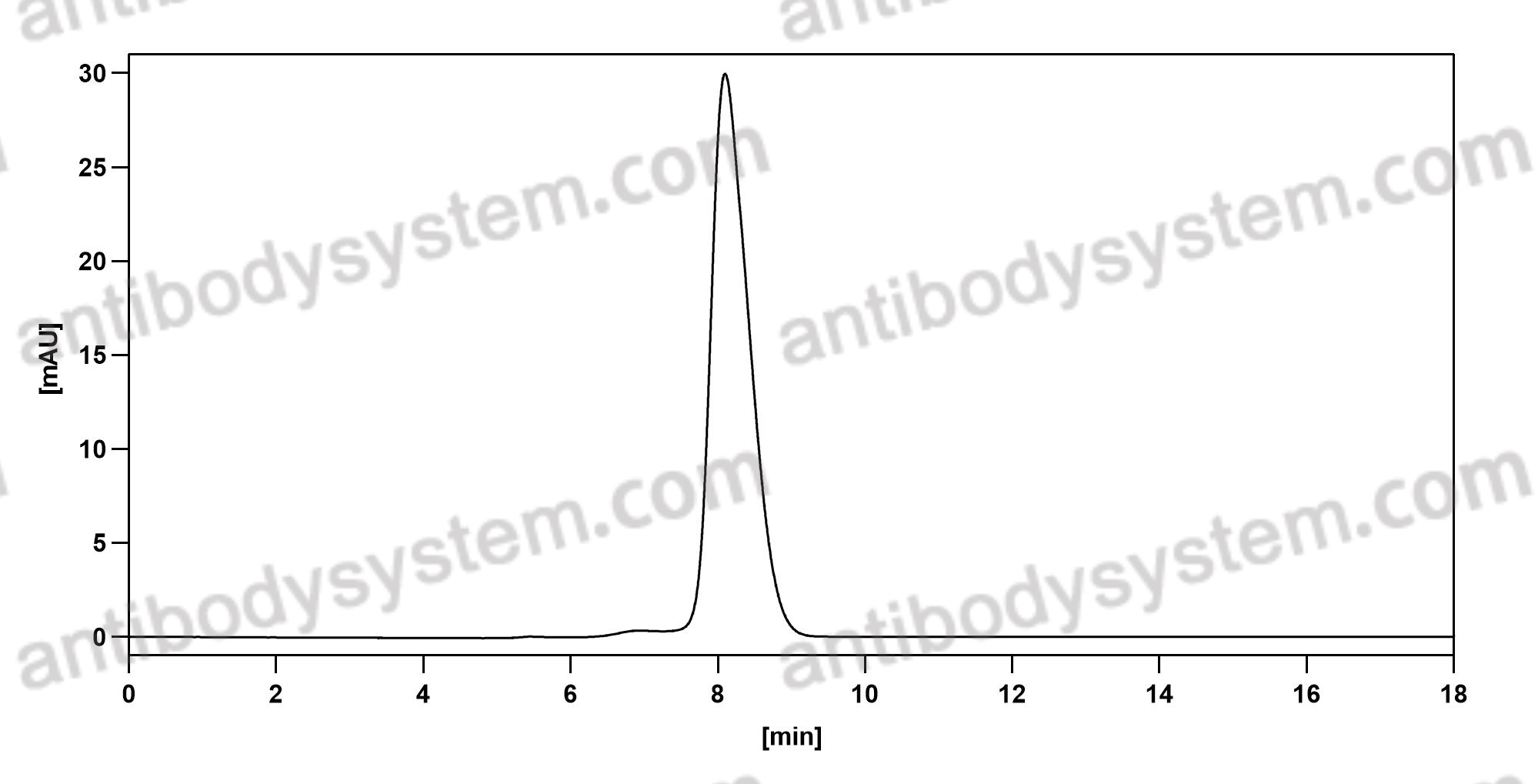
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P05067
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BART, BIIB-037, CAS: 1384260-65-4
Clone ID
Aducanumab
A resurrection of aducanumab for Alzheimer's disease, PMID: 31978357
Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019, PMID: 33135381
Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval, PMID: 32787971
First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer's disease, PMID: 29067304
The antibody aducanumab reduces Aβ plaques in Alzheimer's disease, PMID: 27582220
Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β, PMID: 29686315
Aducanumab and the FDA - where are we now?, PMID: 33442064
Aducanumab reduces Aβ plaques in Alzheimer's disease, PMID: 27739124
Alzheimer disease and aducanumab: adjusting our approach, PMID: 31138932
Questions EMERGE as Biogen claims aducanumab turnaround, PMID: 31784690
Drug treatments in Alzheimer's disease, PMID: 27251914
Impact of Reference and Target Region Selection on Amyloid PET SUV Ratios in the Phase 1b PRIME Study of Aducanumab, PMID: 29777003
Clinical Development of Aducanumab, an Anti-Aβ Human Monoclonal Antibody Being Investigated for the Treatment of Early Alzheimer's Disease, PMID: 29181491
Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice, PMID: 27810931
Post-hoc analysis could give new life to the Alzheimer's drug aducanumab, PMID: 33139929
Open Peer Commentary to "Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE Trials as reported by Biogen December 2019", PMID: 33135288
Addendum: The antibody aducanumab reduces Aβ plaques in Alzheimer's disease, PMID: 28640269
Alzheimer's disease: Recent treatment strategies, PMID: 32941929
Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab, PMID: 33072846
Anti-Aβ Antibody Aducanumab Regulates the Proteome of Senile Plaques and Closely Surrounding Tissue in a Transgenic Mouse Model of Alzheimer's Disease, PMID: 33252074
Antibodies to watch in 2020, PMID: 31847708
The amyloid hypothesis of Alzheimer's disease at 25 years, PMID: 27025652
Aducanumab, PMID: 34424635
Aducanumab: First Approval, PMID: 34324167
Aducanumab, PMID: 34251780
Aducanumab-avwa, PMID: 34268554
From monomer to fibril: Abeta-amyloid binding to Aducanumab antibody studied by molecular dynamics simulation, PMID: 32666627
A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease, PMID: 30610216
Aducanumab: What about the Patient?, PMID: 34322904
Alzheimer's disease: targeting the glutamatergic system, PMID: 32048098
Drug candidates in clinical trials for Alzheimer's disease, PMID: 28720101
Aducanumab for Alzheimer's disease?, PMID: 34226181
Why aducanumab is important, PMID: 34413516
Anti-amyloid-β protein agents for the treatment of Alzheimer's disease: an update on emerging drugs, PMID: 32772738
Kinetic fingerprints differentiate the mechanisms of action of anti-Aβ antibodies, PMID: 32989305
Aducanumab and the certainty of evidence, PMID: 34405228
Pathways Connecting Late-Life Depression and Dementia, PMID: 32231570
Efficacy and safety of anti-amyloid- β immunotherapy for Alzheimer's disease: a systematic review and network meta-analysis, PMID: 29296624
Passive antiamyloid immunotherapy for Alzheimer's disease, PMID: 32040044
The next steps in curing Alzheimer's disease, PMID: 31699316
Lessons Learnt from the Second Generation of Anti-Amyloid Monoclonal Antibodies Clinical Trials, PMID: 33321511
AMDA Position Statement on Aducanumab, PMID: 34456010
The path forward in Alzheimer's disease therapeutics: Reevaluating the amyloid cascade hypothesis, PMID: 31706733
Acute targeting of pre-amyloid seeds in transgenic mice reduces Alzheimer-like pathology later in life, PMID: 33199898
Aducanumab: Appropriate use recommendations, PMID: 34314093
[Aducanumab and Alzheimer's disease: a critical reflection], PMID: 34263875
Aducanumab: look before leaping, PMID: 34413517
Aducanumab, amyloid, and culture wars, PMID: 34413026
The Problem of Aducanumab for the Treatment of Alzheimer Disease, PMID: 34138642
Aducanumab: The first targeted Alzheimer's therapy, PMID: 34234067
Comparison of safety of lecanemab and aducanumab: a real-world disproportionality analysis using the FDA adverse event reporting system., PMID:40520157
Antiamyloid treatment for dementia: concerns outweigh hopes., PMID:40511512
Disease severity across psychiatric disorders is linked to pro-inflammatory cytokines., PMID:40505822
Anti-amyloid antibody equilibrium binding to Aβ aggregates from human Alzheimer disease brain., PMID:40475543
Chimeric antigen receptors discriminate between tau and distinct amyloid-beta species., PMID:40448101
Insights into pathophysiology, biomarkers, and therapeutics in tauopathies: Proceedings of the Tau2024 Global Conference., PMID:40437880
[Prospects for treating Alzheimer's disease]., PMID:40420451
Anti-amyloid immunotherapies for Alzheimer's disease: Administration, side effects, and overall framework., PMID:40413817
Lower baseline amyloid beta burden is associated with greater percent of amyloid beta positron emission tomography reduction and better clinical outcomes in the aducanumab Phase 3 trials ENGAGE and EMERGE in early Alzheimer's disease., PMID:40413110
Aducanumab delivery via focused ultrasound-induced transient blood-brain barrier opening in vivo., PMID:40404732
TRAILBLAZER-ALZ 4: A phase 3 trial comparing donanemab with aducanumab on amyloid plaque clearance in early, symptomatic Alzheimer's disease., PMID:40390253
Comparative efficacy, tolerability, and acceptability of aducanumab, lecanemab, and donanemab with repetitive transcranial magnetic stimulation on cognitive function in mild cognitive impairment and Alzheimer's disease: A systematic review and network meta-analysis., PMID:40386876
Juxtacortical Lesion Precedes Lobar Microbleed During Anti-amyloid β Immunotherapy., PMID:40368787
Cortical microstructural abnormalities in dementia with Lewy bodies and their associations with Alzheimer's disease copathologies., PMID:40355490
The Efficacy of Anti-amyloid Monoclonal Antibodies in Early Alzheimer's Dementia: A Systematic Review., PMID:40346804
Use of Model-Based Meta-Analysis to Inform the Design of Early Clinical Trials of Anti-Amyloid Beta Therapies in Alzheimer's Disease., PMID:40344388
The efficacy and safety of anti-amyloid monoclonal antibody versus acetylcholinesterase inhibitor with an in-depth analysis across genotypes and disease stages: a systematic review and meta-analysis., PMID:40316479
Clinically meaningful benefit and real-world evidence in Alzheimer's disease research and care., PMID:40291121
A real-world pharmacovigilance study of adverse drug reactions associated with lecanemab and aducanumab based on WHO-VigiAccess and FAERS databases., PMID:40290431
Cost-effectiveness analysis of aducanumab versus placebo for patients with mild cognitive impairment and mild Alzheimer's disease., PMID:40250880
The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer's disease based solely on biomarker evidence., PMID:40243238
Lecanemab preferentially binds to smaller aggregates present at early Alzheimer's disease., PMID:40237235
The Importance of Vaccines in Preventing Impending Alzheimer's Epidemic., PMID:40231511
Global experience in brain amyloid imaging., PMID:40222870
Longitudinal Evolution of Posterior Cortical Atrophy: Diagnostic Delays, Overlapping Phenotypes, and Clinical Outcomes., PMID:40198862
Clinical Benefits and Risks of Antiamyloid Antibodies in Sporadic Alzheimer Disease: Systematic Review and Network Meta-Analysis With a Web Application., PMID:40194268
A review of public comments submitted to the Centers for Medicare and Medicaid Services in response to the 2022 National Coverage Decision on treatment for Alzheimer's disease., PMID:40190585
A real-world safety surveillance study of aducanumab through the FDA adverse event reporting system., PMID:40170724
Generalizability of trial criteria on amyloid-lowering therapy against Alzheimer's disease to individuals with mild cognitive impairment or early Alzheimer's disease in the general population., PMID:40122980
Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer's disease: Strategies and research directions., PMID:40118715
Developing Treatment Models for the Delivery of the Antiamyloid Therapy, Lecanemab: Considerations for Implementation of Lecanemab in Healthcare Systems., PMID:40058984
Demographic and clinical characteristics of initial patients receiving amyloid-targeting treatments in the United States after regulatory approval., PMID:40042495
Patient eligibility for amyloid-targeting immunotherapies in Alzheimer's disease., PMID:40011174
Lecanemab for early Alzheimer's disease: Appropriate use recommendations from the French federation of memory clinics., PMID:40011173
Targeting Amyloid Pathology in Early Alzheimer's: The Promise of Donanemab-Azbt., PMID:39998021
Plasma Alzheimer's disease biomarker relationships with incident abnormal amyloid PET., PMID:39989078
Amyloid-β Clearance with Monoclonal Antibodies: Transforming Alzheimer's Treatment., PMID:39980294
Chimeric Antigen Receptors Discriminate Between Tau and Distinct Amyloid-Beta Species., PMID:39974919
[Diagnosis and Treatment of Alzheimer's Disease: Current Update]., PMID:39958494
Correction to: Co-pathology may impact outcomes of amyloid-targeting treatments: clinicopathological results from two patients treated with aducanumab., PMID:39945923
Operationalizing selection criteria for clinical trials in Alzheimer's disease: Biomarker and clinical considerations: Proceedings from the Alzheimer's Association Research Roundtable (AARR) Fall 2021 meeting., PMID:39935618
Drug-induced dementia: a real-world pharmacovigilance study using the FDA Adverse Event Reporting System database., PMID:39882323
Second-generation anti-amyloid monoclonal antibodies for Alzheimer's disease: current landscape and future perspectives., PMID:39865265
Aducanumab in Alzheimer's Disease: A Comparative Study of Its Effects on Dementia and Mild Cognitive Impairment., PMID:39830554
Multi-Targeting Phytochemicals for Alzheimer's Disease., PMID:39815655
Therapeutic Options in Alzheimer's Disease: From Classic Acetylcholinesterase Inhibitors to Multi-Target Drugs with Pleiotropic Activity., PMID:39768263
Safety Concerns in Neurological Clinical Trials: A Challenge That the FDA Must Resolve., PMID:39767824
Mining and analysis of adverse events associated with aducanumab: a real-world study using FDA Adverse Event Reporting System database., PMID:39726994
Overview of Alzheimer's Disease Neuroimaging Initiative and future clinical trials., PMID:39711072
Brain MRI volumetry and atrophy rating scales as predictors of amyloid status and eligibility for anti-amyloid treatment in a real-world memory clinic setting., PMID:39708177