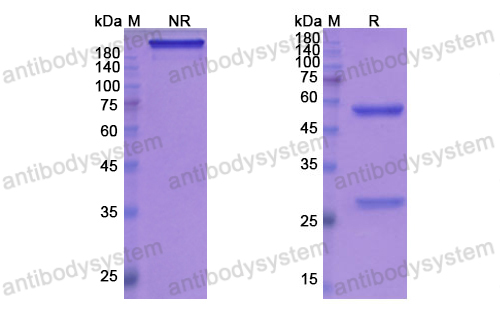
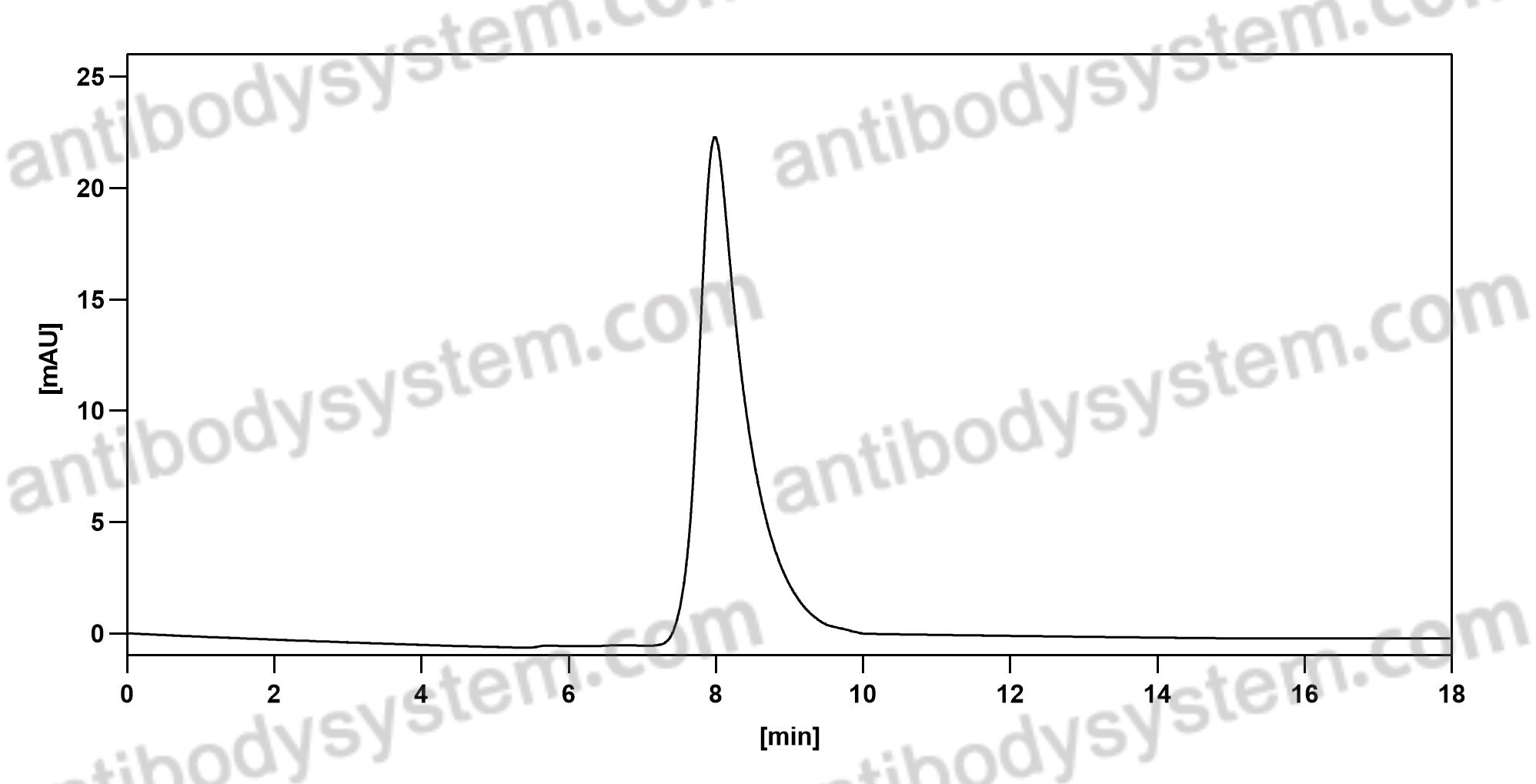
Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval, PMID: 32787971
A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease, PMID: 29221491
Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer's disease: a PET substudy interim analysis, PMID: 31831056
Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β, PMID: 21955818
Gantenerumab for the treatment of Alzheimer's disease, PMID: 22583155
Profile of gantenerumab and its potential in the treatment of Alzheimer's disease, PMID: 24255592
Drug candidates in clinical trials for Alzheimer's disease, PMID: 28720101
Efficacy and safety studies of gantenerumab in patients with Alzheimer's disease, PMID: 25081412
Computational Investigation of Gantenerumab and Crenezumab Recognition of Aβ Fibrils in Alzheimer's Disease Brain Tissue, PMID: 32991803
Anti-amyloid-β protein agents for the treatment of Alzheimer's disease: an update on emerging drugs, PMID: 32772738
Kinetic fingerprints differentiate the mechanisms of action of anti-Aβ antibodies, PMID: 32989305
Passive antiamyloid immunotherapy for Alzheimer's disease, PMID: 32040044
The potential of solanezumab and gantenerumab to prevent Alzheimer's disease in people with inherited mutations that cause its early onset, PMID: 29037101
Lessons Learnt from the Second Generation of Anti-Amyloid Monoclonal Antibodies Clinical Trials, PMID: 33321511
Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β, PMID: 29686315
A Phase I Study to Assess the Effect of Speed of Injection on Pain, Tolerability, and Pharmacokinetics After High-volume Subcutaneous Administration of Gantenerumab in Healthy Volunteers, PMID: 31883703
Reproductive and developmental toxicology studies with gantenerumab in PS2APP transgenic mice, PMID: 28754630
Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab, PMID: 21987394
Thirty-Six-Month Amyloid Positron Emission Tomography Results Show Continued Reduction in Amyloid Burden with Subcutaneous Gantenerumab, PMID: 33336218
A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease, PMID: 34155411
Correction to: A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease, PMID: 30261916
Combined treatment with a BACE inhibitor and anti-Aβ antibody gantenerumab enhances amyloid reduction in APPLondon mice, PMID: 25164658
Amyloid-based immunotherapy for Alzheimer's disease in the time of prevention trials: the way forward, PMID: 24490853
Novel strategies for Alzheimer's disease treatment: An overview of anti-amyloid beta monoclonal antibodies, PMID: 28066098
Alzheimer disease immunotherapeutics: then and now, PMID: 25483498
Is there still any hope for amyloid-based immunotherapy for Alzheimer's disease?, PMID: 24445401
Therapeutic strategies for Alzheimer's disease in clinical trials, PMID: 26721364
Structural aspects of Alzheimer's disease immunotherapy targeted against amyloid-beta peptide, PMID: 29663816
Amyloid-directed monoclonal antibodies for the treatment of Alzheimer's disease: the point of no return?, PMID: 24981190
Emerging drugs to reduce abnormal β-amyloid protein in Alzheimer's disease patients, PMID: 27678025
Can Anti-β-amyloid Monoclonal Antibodies Work in Autosomal Dominant Alzheimer Disease?, PMID: 33575481
Current and Emerging Pharmacological Targets for the Treatment of Alzheimer's Disease, PMID: 31594236
Which are the antibodies to watch in 2013?, PMID: 23254906
Antibodies to watch in 2018, PMID: 29300693
The Alzheimer disease market, PMID: 26388231
Developing Disease-Modifying Treatments in Alzheimer's Disease - A Perspective from Roche and Genentech, PMID: 29181492
[Antibody therapy for Alzheimer's disease], PMID: 22277519
Clinical trials of amyloid-based immunotherapy for Alzheimer's disease: end of beginning or beginning of end?, PMID: 24053611
Critical Appraisal of Amyloid Lowering Agents in AD, PMID: 34110536
Treatments for Alzheimer's disease emerge, PMID: 34353940
Significance of Oligomeric and Fibrillar Species in Amyloidosis: Insights into Pathophysiology and Treatment, PMID: 34443678
Passive immunotherapy for Alzheimer's disease: what have we learned, and where are we headed?, PMID: 23299379
Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer's disease, PMID: 33831607
Antibody drugs for Alzheimer's show glimmers of promise, PMID: 26223602
Comparing the efficacy and neuroinflammatory potential of three anti-abeta antibodies, PMID: 26433971
SAR228810: an antibody for protofibrillar amyloid β peptide designed to reduce the risk of amyloid-related imaging abnormalities (ARIA), PMID: 30486882
Amyloid-Related Imaging Abnormalities (ARIA) in Immunotherapy Trials for Alzheimer's Disease: Need for Prognostic Biomarkers?, PMID: 27031492
Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer's Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression, PMID: 34198582
Aducanumab produced a clinically meaningful benefit in association with amyloid lowering, PMID: 33971962
Immunohistochemical evaluation of a trial of gantenerumab or solanezumab in dominantly inherited Alzheimer disease., PMID:40459787
Trends in Research on Gantenerumab for Alzheimer's Disease: A Bibliometric and Thematic Analysis., PMID:40390221
The Efficacy of Anti-amyloid Monoclonal Antibodies in Early Alzheimer's Dementia: A Systematic Review., PMID:40346804
Japanese participant data from three gantenerumab trials in early Alzheimer's disease., PMID:40302041
The science does not yet support regulatory approval of amyloid-targeting therapies for Alzheimer's disease based solely on biomarker evidence., PMID:40243238
Lecanemab preferentially binds to smaller aggregates present at early Alzheimer's disease., PMID:40237235
Donanemab: Appropriate use recommendations., PMID:40155270
Safety and efficacy of long-term gantenerumab treatment in dominantly inherited Alzheimer's disease: an open-label extension of the phase 2/3 multicentre, randomised, double-blind, placebo-controlled platform DIAN-TU trial., PMID:40120616
Lecanemab for early Alzheimer's disease: Appropriate use recommendations from the French federation of memory clinics., PMID:40011173
Safety and efficacy of long-term gantenerumab treatment in dominantly inherited Alzheimer's disease: an open label extension of the phase 2/3 multicentre, randomised, double-blind, placebo-controlled platform DIAN-TU Trial., PMID:39974075
Biomarker treatment effects in two phase 3 trials of gantenerumab., PMID:39887500
Second-generation anti-amyloid monoclonal antibodies for Alzheimer's disease: current landscape and future perspectives., PMID:39865265
Favorable long-term cognitive outcomes following recurrent ARIA linked to amyloid-lowering therapies: two cases., PMID:39853424
Long-term safety of gantenerumab in participants with Alzheimer's disease: A phase III, open-label extension study (SCarlet RoAD)., PMID:39686620
Amyloid-Related Imaging Abnormalities (ARIA) in Clinical Trials of Gantenerumab in Early Alzheimer Disease., PMID:39556389
Gantenerumab in Dominantly Inherited Alzheimer Disease., PMID:39527042
Gantenerumab in Dominantly Inherited Alzheimer Disease-Reply., PMID:39527036
2024 AA criteria for Alzheimer's disease diagnosis: Mainly anchored at Aβ not tau., PMID:39470319
A systematic review of the efficacy and safety of anti-amyloid beta monoclonal antibodies in treatment of Alzheimer's disease., PMID:39432414
Alzheimer's Disease Linkage to Real-World Evidence (AD-LINE) Study: Linking Claims Data to Phase 3 GRADUATE Study of Gantenerumab., PMID:39350370
Limitations and potential strategies of immune checkpoint blockade in age-related neurodegenerative disorders., PMID:39313800
Benefits and risks of FDA-approved amyloid-targeting antibodies for treatment of early Alzheimer's disease: Navigating clinician-patient engagement., PMID:39306695
Association of Resilience-Related Life Experiences on Variability on Age of Onset in Dominantly Inherited Alzheimer Disease., PMID:39270149
Long-Term Safety of Gantenerumab in Participants with Alzheimer's Disease: A Phase III, Double-Blind, and Open-Label Extension Study (Marguerite RoAD)., PMID:39177596
Scenarios for the long-term efficacy of amyloid-targeting therapies in the context of the natural history of Alzheimer's disease., PMID:39073291
Predicting continuous amyloid PET values with CSF tau phosphorylation occupancies., PMID:39041391
Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders., PMID:38928395
Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS., PMID:38890531
Gantenerumab for early Alzheimer's disease: a systematic review and meta-analysis., PMID:38879828
Beyond lecanemab: Examining Phase III potential in Alzheimer's therapeutics., PMID:38868475
Immunotherapy in Alzheimer's disease: focusing on the efficacy of gantenerumab on amyloid-β clearance and cognitive decline., PMID:38767981
A Review of Recent Advances in the Management of Alzheimer's Disease., PMID:38756263
Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities., PMID:38740836
Passive immunotherapy for Alzheimer's disease: challenges & future directions., PMID:38715084
CSF neurofilament light chain profiling and quantitation in neurological diseases., PMID:38707707
Dominantly Inherited Alzheimer Network Trials Unit (DIAN-TU): Trial Satisfaction and Attitudes towards Future Clinical Trials., PMID:38706272
At-Home Administration of Gantenerumab by Care Partners to People with Early Alzheimer's Disease: Feasibility, Safety and Pharmacodynamic Impact., PMID:38706270
Lessons learned from the failure of solanezumab as a prospective treatment strategy for Alzheimer's disease., PMID:38685682
Downstream Biomarker Effects of Gantenerumab or Solanezumab in Dominantly Inherited Alzheimer Disease: The DIAN-TU-001 Randomized Clinical Trial., PMID:38683602
[The monoclonal antibody gantenerumab in the treatment of early Alzheimer's disease]., PMID:38630163
What is Alzheimer's disease? An analysis of nosological perspectives from the 20th and 21st centuries., PMID:38618742
Deconstructing pathological tau by biological process in early stages of Alzheimer disease: a method for quantifying tau spatial spread in neuroimaging., PMID:38552342
Comparative Efficacy and Safety of Monoclonal Antibodies for Cognitive Decline in Patients with Alzheimer's Disease: A Systematic Review and Network Meta-Analysis., PMID:38429615
Gantenerumab in Early Alzheimer's Disease. Reply., PMID:38416440
Gantenerumab in Early Alzheimer's Disease., PMID:38416439
Prognostic and Predictive Factors in Early Alzheimer's Disease: A Systematic Review., PMID:38405341
Examining amyloid reduction as a surrogate endpoint through latent class analysis using clinical trial data for dominantly inherited Alzheimer's disease., PMID:38400532
Bayesian meta-analysis of phase 3 results of aducanumab, lecanemab, donanemab, and high-dose gantenerumab in prodromal and mild Alzheimer's disease., PMID:38389855
Risk factors in developing amyloid related imaging abnormalities (ARIA) and clinical implications., PMID:38312931
Performance of the Lumipulse plasma Aβ42/40 and pTau181 immunoassays in the detection of amyloid pathology., PMID:38304322