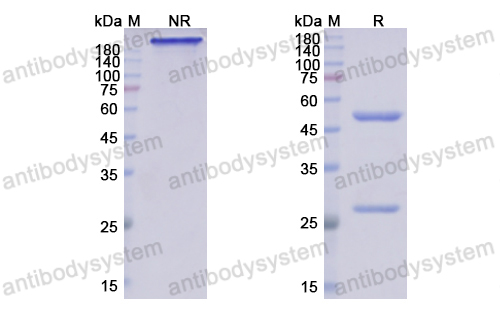
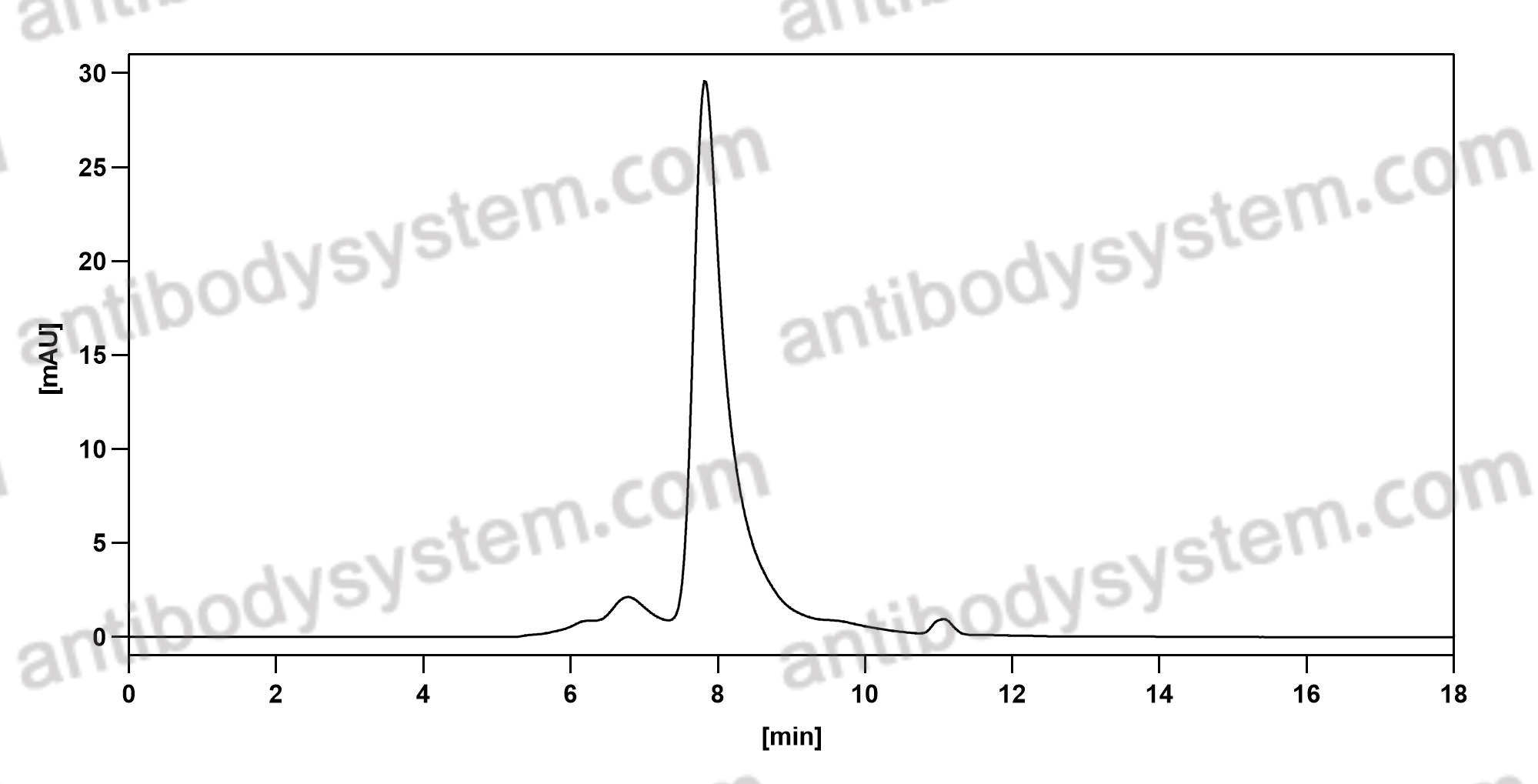
The amyloid hypothesis of Alzheimer's disease at 25 years, PMID: 27025652
Drug candidates in clinical trials for Alzheimer's disease, PMID: 28720101
Pharmacokinetics and pharmacodynamic effect of crenezumab on plasma and cerebrospinal fluid beta-amyloid in patients with mild-to-moderate Alzheimer's disease, PMID: 31969177
Passive antiamyloid immunotherapy for Alzheimer's disease, PMID: 32040044
Pathways Connecting Late-Life Depression and Dementia, PMID: 32231570
Safety, Tolerability, and Pharmacokinetics of Crenezumab in Patients with Mild-to-Moderate Alzheimer's Disease Treated with Escalating Doses for up to 133 Weeks, PMID: 32568196
Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid, PMID: 31168802
Lessons Learnt from the Second Generation of Anti-Amyloid Monoclonal Antibodies Clinical Trials, PMID: 33321511
Characterization of the selective in vitro and in vivo binding properties of crenezumab to oligomeric Aβ, PMID: 31787113
Structure of Crenezumab Complex with Aβ Shows Loss of β-Hairpin, PMID: 27996029
ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease, PMID: 29695589
Computational Investigation of Gantenerumab and Crenezumab Recognition of Aβ Fibrils in Alzheimer's Disease Brain Tissue, PMID: 32991803
Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE), PMID: 30231896
The Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer's disease, including a placebo-treated noncarrier cohort, PMID: 29955659
Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N‑truncated Abeta in sporadic Alzheimer disease cases and mouse models, PMID: 26467270
Mechanisms of recognition of amyloid-β (Aβ) monomer, oligomer, and fibril by homologous antibodies, PMID: 28924036
Do current therapeutic anti-Aβ antibodies for Alzheimer's disease engage the target?, PMID: 24803227
Safety, Tolerability, and Pharmacokinetics of High-Volume Subcutaneous Crenezumab, With and Without Recombinant Human Hyaluronidase in Healthy Volunteers, PMID: 34347883
Mechanistic Modeling of Soluble Aβ Dynamics and Target Engagement in the Brain by Anti-Aβ mAbs in Alzheimer's Disease, PMID: 32116192
N-terminal heterogeneity of parenchymal and vascular amyloid-β deposits in Alzheimer's disease, PMID: 32497293
Adherence/Retention Alzheimer's Prevention Initiative Colombia Plan, PMID: 30090848
Therapeutic strategies for Alzheimer's disease in clinical trials, PMID: 26721364
Amyloid-based immunotherapy for Alzheimer's disease in the time of prevention trials: the way forward, PMID: 24490853
Novel strategies for Alzheimer's disease treatment: An overview of anti-amyloid beta monoclonal antibodies, PMID: 28066098
Alzheimer disease immunotherapeutics: then and now, PMID: 25483498
Is there still any hope for amyloid-based immunotherapy for Alzheimer's disease?, PMID: 24445401
Structural aspects of Alzheimer's disease immunotherapy targeted against amyloid-beta peptide, PMID: 29663816
Developing Disease-Modifying Treatments in Alzheimer's Disease - A Perspective from Roche and Genentech, PMID: 29181492
Amyloid-directed monoclonal antibodies for the treatment of Alzheimer's disease: the point of no return?, PMID: 24981190
Baseline demographic, clinical, and cognitive characteristics of the Alzheimer's Prevention Initiative (API) Autosomal-Dominant Alzheimer's Disease Colombia Trial, PMID: 32418361
Passive Immunotherapies Targeting Amyloid Beta and Tau Oligomers in Alzheimer's Disease, PMID: 31647950
Molecular basis for mid-region amyloid-β capture by leading Alzheimer's disease immunotherapies, PMID: 25880481
Can Anti-β-amyloid Monoclonal Antibodies Work in Autosomal Dominant Alzheimer Disease?, PMID: 33575481
Critical Appraisal of Amyloid Lowering Agents in AD, PMID: 34110536
Passive immunotherapy for Alzheimer's disease: what have we learned, and where are we headed?, PMID: 23299379
The Value of Pre-Screening in the Alzheimer's Prevention Initiative (API) Autosomal Dominant Alzheimer's Disease Trial, PMID: 29405233
Rational affinity maturation of anti-amyloid antibodies with high conformational and sequence specificity, PMID: 33675750
Alzheimer's drugs take a new tack, PMID: 22962697
Pioneering Alzheimer's study in Colombia zeroes in on enigmatic protein, PMID: 29595782
Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer's disease, PMID: 33831607
Colombia at the centre of preclinical AD research, PMID: 22710749
Comparing the efficacy and neuroinflammatory potential of three anti-abeta antibodies, PMID: 26433971
LIFTING THE CURSE OF ALZHEIMER'S, PMID: 26336711
Alzheimer's research. Stopping Alzheimer's before it starts, PMID: 22903991
Systematic in silico analysis of clinically tested drugs for reducing amyloid-beta plaque accumulation in Alzheimer's disease, PMID: 33938131
Genentech's Alzheimer's antibody trial to study disease prevention, PMID: 22871696
Sting of Alzheimer's failures offset by upcoming prevention trials, PMID: 22935790
US government sets out Alzheimer's plan, PMID: 22622544
The Efficacy of Anti-amyloid Monoclonal Antibodies in Early Alzheimer's Dementia: A Systematic Review., PMID:40346804
Second-generation anti-amyloid monoclonal antibodies for Alzheimer's disease: current landscape and future perspectives., PMID:39865265
A systematic review of the efficacy and safety of anti-amyloid beta monoclonal antibodies in treatment of Alzheimer's disease., PMID:39432414
Limitations and potential strategies of immune checkpoint blockade in age-related neurodegenerative disorders., PMID:39313800
Strategies to promote contraception use by female volunteers in Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease (API ADAD) Colombia trial., PMID:39143683
Engineered Antibodies to Improve Efficacy against Neurodegenerative Disorders., PMID:38928395
A Review of Recent Advances in the Management of Alzheimer's Disease., PMID:38756263
Amyloid-beta antibody binding to cerebral amyloid angiopathy fibrils and risk for amyloid-related imaging abnormalities., PMID:38740836
Native ion mobility-mass spectrometry reveals the binding mechanisms of anti-amyloid therapeutic antibodies., PMID:38723181
Passive immunotherapy for Alzheimer's disease: challenges & future directions., PMID:38715084
Evaluation of Patient-Centric Sample Collection Technologies for Pharmacokinetic Assessment of Large and Small Molecules., PMID:38671563
Correction: Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncted Abeta in sporadic Alzheimer disease cases and mouse models., PMID:38526686
Alzheimer's disease and clinical trials., PMID:38491747
Re-Engineering Therapeutic Anti-Aβ Monoclonal Antibody to Target Amyloid Light Chain., PMID:38338870
Development of amyloid beta-directed antibodies against Alzheimer's disease: Twists and turns., PMID:38220210
Patients with geriatric syndromes and anti-amyloid therapies: lack of consideration? An exploratory analysis of the literature., PMID:37881360
Validation of low-volume sampling devices for pharmacokinetic analysis: technical and logistical challenges and solutions., PMID:37855111
Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer's Disease Therapeutic Antibodies., PMID:37176076
Longitudinal Exposure-Response Modeling of Multiple Indicators of Alzheimer's Disease Progression., PMID:36946448
Investigational treatments for neurodegenerative diseases caused by inheritance of gene mutations: lessons from recent clinical trials., PMID:36751779
The Use of Episodic Memory Tests for Screening in Clinical Trials for Early Alzheimer's Disease: A Comparison of the Free and Cued Selective Reminding Test (FCSRT) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)., PMID:36641609
A combined physiologically-based pharmacokinetic and quantitative systems pharmacology model for modeling amyloid aggregation in Alzheimer's disease., PMID:36632701
A public resource of baseline data from the Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease Trial., PMID:36373344
Quantitative systems pharmacology model of the amyloid pathway in Alzheimer's disease: Insights into the therapeutic mechanisms of clinical candidates., PMID:36281062
Evaluating the Safety and Efficacy of Crenezumab vs Placebo in Adults With Early Alzheimer Disease: Two Phase 3 Randomized Placebo-Controlled Trials., PMID:36121669
ACU193: An Immunotherapeutic Poised to Test the Amyloid β Oligomer Hypothesis of Alzheimer's Disease., PMID:35557606
Safety, Tolerability, and Pharmacokinetics of High-Volume Subcutaneous Crenezumab, With and Without Recombinant Human Hyaluronidase in Healthy Volunteers., PMID:34347883
Critical Appraisal of Amyloid Lowering Agents in AD., PMID:34110536
Systematic in silico analysis of clinically tested drugs for reducing amyloid-beta plaque accumulation in Alzheimer's disease., PMID:33938131
Effects of monoclonal antibodies against amyloid-β on clinical and biomarker outcomes and adverse event risks: A systematic review and meta-analysis of phase III RCTs in Alzheimer's disease., PMID:33831607
Rational affinity maturation of anti-amyloid antibodies with high conformational and sequence specificity., PMID:33675750
Can Anti-β-amyloid Monoclonal Antibodies Work in Autosomal Dominant Alzheimer Disease?, PMID:33575481
Lessons Learnt from the Second Generation of Anti-Amyloid Monoclonal Antibodies Clinical Trials., PMID:33321511
Computational Investigation of Gantenerumab and Crenezumab Recognition of Aβ Fibrils in Alzheimer's Disease Brain Tissue., PMID:32991803
Passive immunotherapies targeting Aβ and tau in Alzheimer's disease., PMID:32682954
Safety, Tolerability, and Pharmacokinetics of Crenezumab in Patients with Mild-to-Moderate Alzheimer's Disease Treated with Escalating Doses for up to 133 Weeks., PMID:32568196
N-terminal heterogeneity of parenchymal and vascular amyloid-β deposits in Alzheimer's disease., PMID:32497293
Baseline demographic, clinical, and cognitive characteristics of the Alzheimer's Prevention Initiative (API) Autosomal-Dominant Alzheimer's Disease Colombia Trial., PMID:32418361
Pathways Connecting Late-Life Depression and Dementia., PMID:32231570
Mechanistic Modeling of Soluble Aβ Dynamics and Target Engagement in the Brain by Anti-Aβ mAbs in Alzheimer's Disease., PMID:32116192
Passive antiamyloid immunotherapy for Alzheimer's disease., PMID:32040044
Pharmacokinetics and pharmacodynamic effect of crenezumab on plasma and cerebrospinal fluid beta-amyloid in patients with mild-to-moderate Alzheimer's disease., PMID:31969177
Characterization of the selective in vitro and in vivo binding properties of crenezumab to oligomeric Aβ., PMID:31787113
Passive Immunotherapies Targeting Amyloid Beta and Tau Oligomers in Alzheimer's Disease., PMID:31647950
Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid., PMID:31168802
Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE)., PMID:30231896
Adherence/Retention Alzheimer's Prevention Initiative Colombia Plan., PMID:30090848
The Alzheimer's Prevention Initiative Autosomal-Dominant Alzheimer's Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer's disease, including a placebo-treated noncarrier cohort., PMID:29955659
ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease., PMID:29695589
Structural aspects of Alzheimer's disease immunotherapy targeted against amyloid-beta peptide., PMID:29663816