Catalog No.
DHB86911
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
HER1, Receptor tyrosine-protein kinase erbB-1, Proto-oncogene c-ErbB-1, Epidermal growth factor receptor, EGFR, ERBB1, ERBB
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
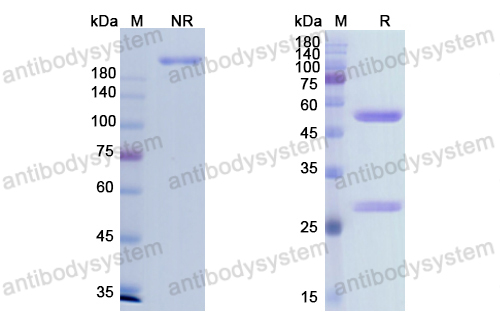
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P00533
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
GA201, RG7160, RO5083945, CAS: 959963-46-3
Clone ID
Imgatuzumab
Diagnostic performance of COVID-19 serology assays, PMID: 32342927
Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines, PMID: 28405498
Premedication and Chemotherapy Agents do not Impair Imgatuzumab (GA201)-Mediated Antibody-Dependent Cellular Cytotoxicity and Combination Therapies Enhance Efficacy, PMID: 26581243
ADCC responses and blocking of EGFR-mediated signaling and cell growth by combining the anti-EGFR antibodies imgatuzumab and cetuximab in NSCLC cells, PMID: 28467975
Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab, PMID: 27602494
An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma, PMID: 28950289
Open-label, multicentre expansion cohort to evaluate imgatuzumab in pre-treated patients with KRAS-mutant advanced colorectal carcinoma, PMID: 24262587
Discontinued in 2013: oncology drugs, PMID: 25315907
Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy, PMID: 24496456
RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation, PMID: 23780344
GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab, PMID: 23209031
Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors, PMID: 21900113
Diagnostic performance of COVID-19 serology assays., PMID:32342927
An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma., PMID:28950289
ADCC responses and blocking of EGFR-mediated signaling and cell growth by combining the anti-EGFR antibodies imgatuzumab and cetuximab in NSCLC cells., PMID:28467975
Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines., PMID:28405498
Extracellular domain shedding influences specific tumor uptake and organ distribution of the EGFR PET tracer 89Zr-imgatuzumab., PMID:27602494
Premedication and Chemotherapy Agents do not Impair Imgatuzumab (GA201)-Mediated Antibody-Dependent Cellular Cytotoxicity and Combination Therapies Enhance Efficacy., PMID:26581243
Discontinued in 2013: oncology drugs., PMID:25315907
Glyco-engineered anti-EGFR mAb elicits ADCC by NK cells from colorectal cancer patients irrespective of chemotherapy., PMID:24496456
Open-label, multicentre expansion cohort to evaluate imgatuzumab in pre-treated patients with KRAS-mutant advanced colorectal carcinoma., PMID:24262587
RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation., PMID:23780344
GA201 (RG7160): a novel, humanized, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab., PMID:23209031
Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors., PMID:21900113