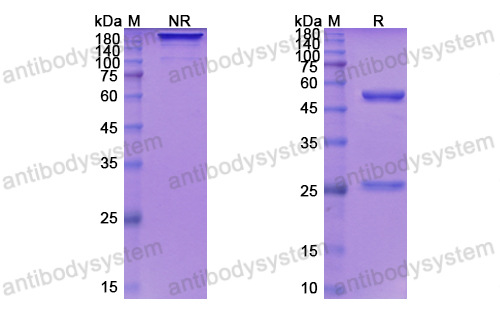
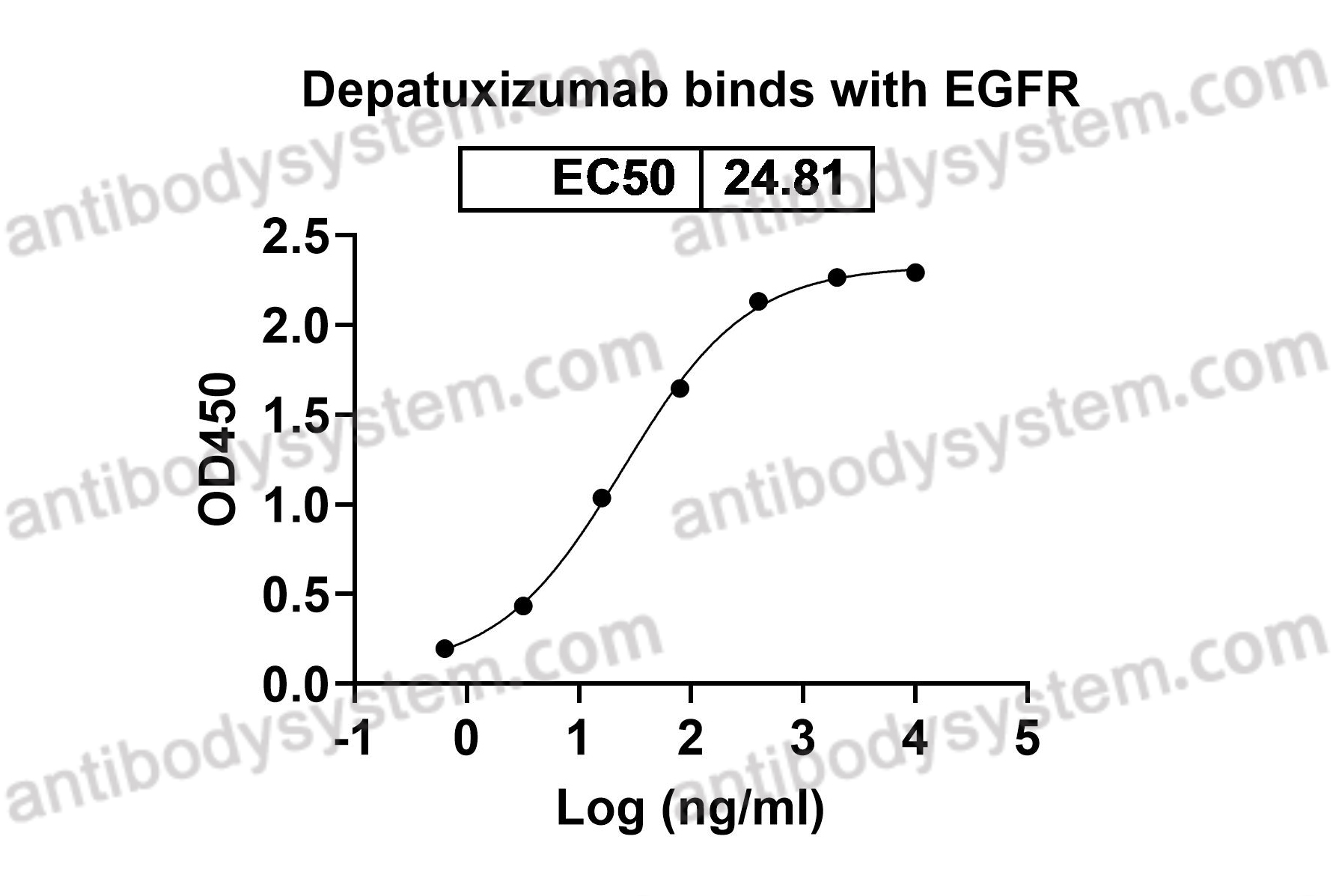
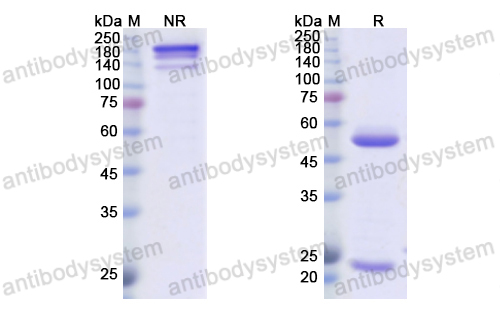
Depatuxizumab Mafodotin (ABT-414)-induced Glioblastoma Cell Death Requires EGFR Overexpression, but not EGFR Y1068 Phosphorylation, PMID: 32371586
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: Retraction, PMID: 30102618
Antibodies to watch in 2019, PMID: 30516432
How we treat glioblastoma, PMID: 31297242
Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial, PMID: 29982805
INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma, PMID: 31747009
Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma, PMID: 29077941
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: [RETRACTED], PMID: 29794826
Efficacy and safety results of depatuxizumab mafodotin (ABT-414) in patients with advanced solid tumors likely to overexpress epidermal growth factor receptor, PMID: 29533458
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate, PMID: 33201054
An Integrated Population Pharmacokinetic Model Versus Individual Models of Depatuxizumab Mafodotin, an Anti-EGFR Antibody Drug Conjugate, in Patients With Solid Tumors Likely to Overexpress EGFR, PMID: 30990907
Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study, PMID: 29075855
Depatuxizumab Mafodotin (Depatux-M) Plus Temozolomide in Recurrent Glioblastoma Patients: Real-World Experience from a Multicenter Study of Italian Association of Neuro-Oncology (AINO), PMID: 34204877
A troublesome burden, the amplification of EGFR in glioblastoma!, PMID: 32144420
Impact of depatuxizumab mafodotin on health-related quality of life and neurological functioning in the phase II EORTC 1410/INTELLANCE 2 trial for EGFR-amplified recurrent glioblastoma, PMID: 33601293
Targeting Multiple EGFR-expressing Tumors with a Highly Potent Tumor-selective Antibody-Drug Conjugate, PMID: 32847977
Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments, PMID: 29116596
Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study, PMID: 33154952
Characterization of ABBV-221, a Tumor-Selective EGFR-Targeting Antibody Drug Conjugate, PMID: 29483208
Corneal side effects induced by EGFR-inhibitor antibody-drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study, PMID: 32550861
Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma, PMID: 34050676
Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody, PMID: 25731184
Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma, PMID: 29449271
A Phase 1 and Biodistribution Study of ABT-806i, an 111 In-Radiolabeled Conjugate of the Tumor-Specific Anti-EGFR Antibody ABT-806, PMID: 33509972
Population Pharmacokinetics of ABT-806, an Investigational Anti-Epidermal Growth Factor Receptor (EGFR) Monoclonal Antibody, in Advanced Solid Tumor Types Likely to Either Over-Express Wild-Type EGFR or Express Variant III Mutant EGFR, PMID: 25761639
Veliparib in Combination With Platinum-Based Chemotherapy for First-Line Treatment of Advanced Squamous Cell Lung Cancer: A Randomized, Multicenter Phase III Study, PMID: 34436928
Bystander Effects, Pharmacokinetics, and Linker-Payload Stability of EGFR-Targeting Antibody-Drug Conjugates Losatuxizumab Vedotin and Depatux-M in Glioblastoma Models., PMID:38743766
Focus on current and emerging treatment options for glioma: A comprehensive review., PMID:38689623
Efficacy of depatuxizumab mafodotin (ABT-414) in preclinical models of head and neck cancer., PMID:38375733
A Phase 3b Study for Management of Ocular Side Effects in Patients with Epidermal Growth Factor Receptor-Amplified Glioblastoma Receiving Depatuxizumab Mafodotin., PMID:37257422
Understanding the activity of antibody-drug conjugates in primary and secondary brain tumours., PMID:37085569
Convection enhanced delivery of EGFR targeting antibody-drug conjugates Serclutamab talirine and Depatux-M in glioblastoma patient-derived xenografts., PMID:36071925
Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial., PMID:35849035
Characterization and Potential Mitigation of Corneal Effects in Nonclinical Toxicology Studies in Animals Administered Depatuxizumab Mafodotin., PMID:35537481
Safety and efficacy of depatuxizumab mafodotin in Japanese patients with malignant glioma: A nonrandomized, phase 1/2 trial., PMID:34609773
Ocular surface toxicity of depatuxizumab mafoditin (ABT-414): case reports., PMID:34586240
Tumor volumes as a predictor of response to the anti-EGFR antibody drug conjugate depatuxizumab mafadotin., PMID:34549181
Veliparib in Combination With Platinum-Based Chemotherapy for First-Line Treatment of Advanced Squamous Cell Lung Cancer: A Randomized, Multicenter Phase III Study., PMID:34436928
Depatuxizumab Mafodotin (Depatux-M) Plus Temozolomide in Recurrent Glioblastoma Patients: Real-World Experience from a Multicenter Study of Italian Association of Neuro-Oncology (AINO)., PMID:34204877
Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma., PMID:34050676
Impact of depatuxizumab mafodotin on health-related quality of life and neurological functioning in the phase II EORTC 1410/INTELLANCE 2 trial for EGFR-amplified recurrent glioblastoma., PMID:33601293
Synergistic therapeutic benefit by combining the antibody drug conjugate, depatux-m with temozolomide in pre-clinical models of glioblastoma with overexpression of EGFR., PMID:33517558
A Phase 1 and Biodistribution Study of ABT-806i, an 111In-Radiolabeled Conjugate of the Tumor-Specific Anti-EGFR Antibody ABT-806., PMID:33509972
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate., PMID:33201054
Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study., PMID:33154952
Targeting Multiple EGFR-expressing Tumors with a Highly Potent Tumor-selective Antibody-Drug Conjugate., PMID:32847977
Corneal side effects induced by EGFR-inhibitor antibody-drug conjugate ABT-414 in patients with recurrent glioblastoma: a prospective clinical and confocal microscopy study., PMID:32550861
Depatuxizumab Mafodotin (ABT-414)-induced Glioblastoma Cell Death Requires EGFR Overexpression, but not EGFRY1068 Phosphorylation., PMID:32371586
A phase 1 study evaluating safety and pharmacokinetics of losatuxizumab vedotin (ABBV-221), an anti-EGFR antibody-drug conjugate carrying monomethyl auristatin E, in patients with solid tumors likely to overexpress EGFR., PMID:32189093
A troublesome burden, the amplification of EGFR in glioblastoma!, PMID:32144420
INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma., PMID:31747009
How we treat glioblastoma., PMID:31297242
An Integrated Population Pharmacokinetic Model Versus Individual Models of Depatuxizumab Mafodotin, an Anti-EGFR Antibody Drug Conjugate, in Patients With Solid Tumors Likely to Overexpress EGFR., PMID:30990907
Antibodies to watch in 2019., PMID:30516432
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: Retraction., PMID:30102618
Safety and efficacy of depatuxizumab mafodotin + temozolomide in patients with EGFR-amplified, recurrent glioblastoma: results from an international phase I multicenter trial., PMID:29982805
Clinical and Histological Characterization of Toxic Keratopathy From Depatuxizumab Mafodotin (ABT-414), an Antibody-Drug Conjugate: [RETRACTED]., PMID:29794826
Efficacy and safety results of depatuxizumab mafodotin (ABT-414) in patients with advanced solid tumors likely to overexpress epidermal growth factor receptor., PMID:29533458
Characterization of ABBV-221, a Tumor-Selective EGFR-Targeting Antibody Drug Conjugate., PMID:29483208
Targeted Therapies for Targeted Populations: Anti-EGFR Treatment for EGFR-Amplified Gastroesophageal Adenocarcinoma., PMID:29449271
Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments., PMID:29116596
Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma., PMID:29077941
Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study., PMID:29075855
Population Pharmacokinetics of ABT-806, an Investigational Anti-Epidermal Growth Factor Receptor (EGFR) Monoclonal Antibody, in Advanced Solid Tumor Types Likely to Either Over-Express Wild-Type EGFR or Express Variant III Mutant EGFR., PMID:25761639
Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody., PMID:25731184