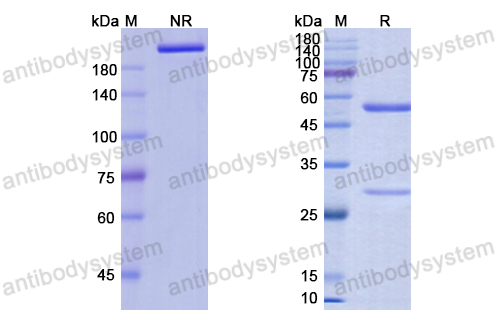
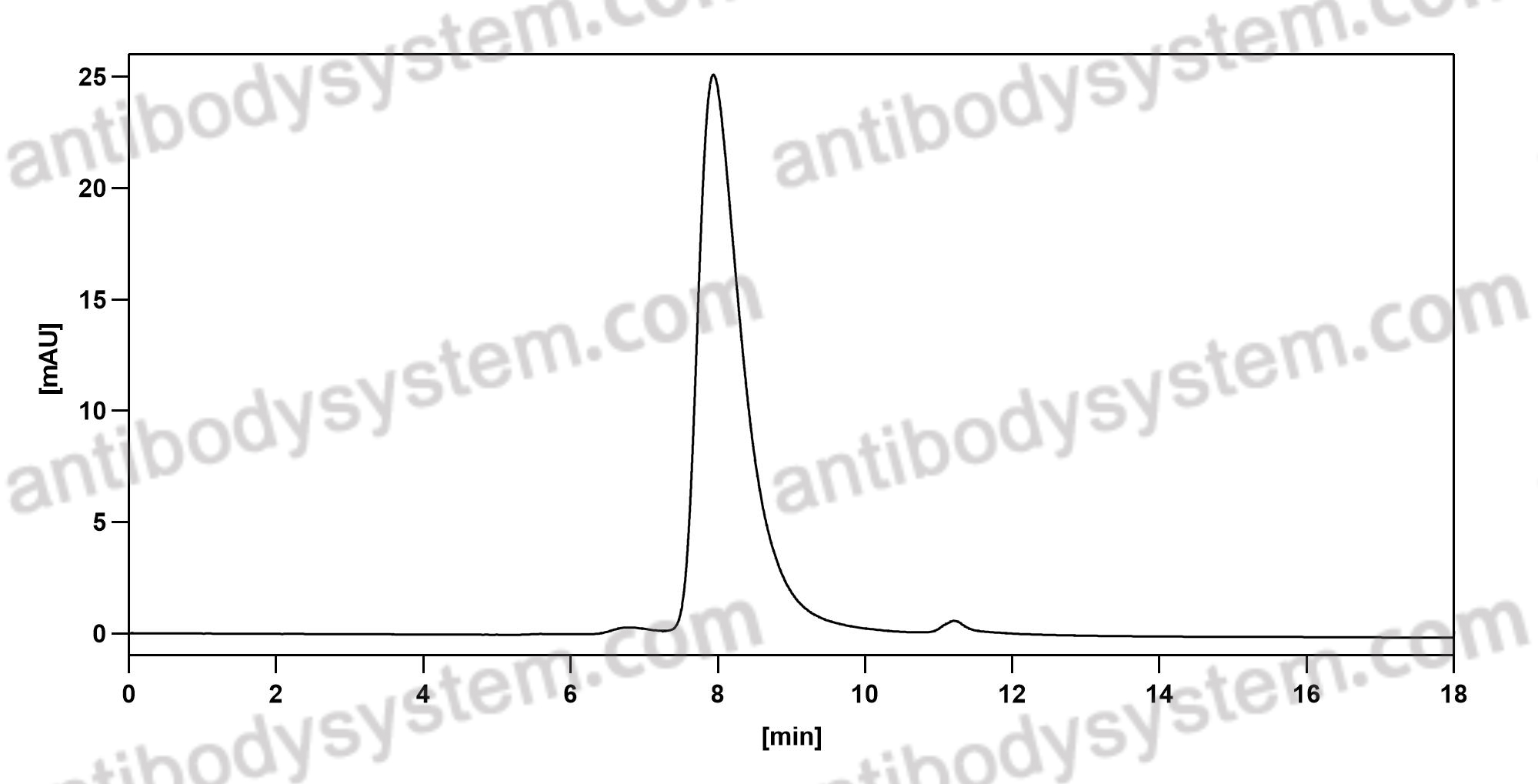
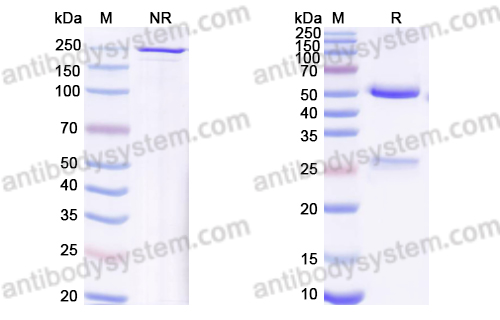
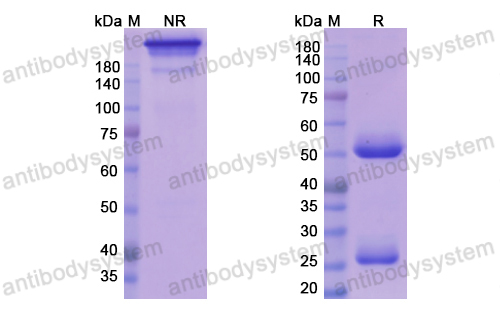
Trial of Anifrolumab in Active Systemic Lupus Erythematosus, PMID: 31851795
Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus, PMID: 28130918
Anifrolumab, PMID: 32310439
Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials, PMID: 32814461
Anifrolumab in systemic lupus erythematosus: current knowledge and future considerations, PMID: 32237942
Anifrolumab in Systemic Lupus Erythematosus, PMID: 32320576
Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date, PMID: 31190735
Anifrolumab in Systemic Lupus Erythematosus. Reply, PMID: 32320577
Antibodies to watch in 2020, PMID: 31847708
Long-Term Safety and Efficacy of Anifrolumab in Adults With Systemic Lupus Erythematosus: Results of a Phase II Open-Label Extension Study, PMID: 33225631
[Anifrolumab in systemic lupus erythematosus], PMID: 29411096
Anifrolumab for the treatment of active systemic lupus erythematosus: a meta-analysis of randomized controlled trials, PMID: 33216191
State-of-the-art treatment of systemic lupus erythematosus, PMID: 32134201
Positive results for anifrolumab in phase III SLE trial, PMID: 32020079
Anifrolumab, PMID: 34406725
Cutaneous Lupus Erythematosus: Progress and Challenges, PMID: 32248318
Safety and tolerability of anifrolumab, a monoclonal antibody targeting type I interferon receptor, in Japanese patients with systemic lupus erythematosus: A multicenter, phase 2, open-label study, PMID: 30793642
Characterisation of anifrolumab, a fully human anti-interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus, PMID: 29644082
Anifrolumab effects on rash and arthritis: impact of the type I interferon gene signature in the phase IIb MUSE study in patients with systemic lupus erythematosus, PMID: 30588322
Efficacy of anifrolumab in systemic lupus erythematosus: a critical analysis of the TULIP trials, PMID: 32493153
Safety, tolerability and pharmacokinetics of subcutaneous and intravenous anifrolumab in healthy volunteers, PMID: 29644080
Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE, PMID: 30538817
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?, PMID: 28765121
Systemic lupus erythematosus, PMID: 29224671
Kidney outcomes for children with lupus nephritis, PMID: 32725543
Treatment of cutaneous lupus erythematosus: current approaches and future strategies, PMID: 32141953
Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-α receptor 1 antibody, PMID: 25606664
Biologics in the treatment of Sjogren's syndrome, systemic lupus erythematosus, and lupus nephritis, PMID: 33002950
Anifrolumab reduces flare rates in patients with moderate to severe systemic lupus erythematosus, PMID: 33977796
B Cell Therapy in Systemic Lupus Erythematosus: From Rationale to Clinical Practice, PMID: 32754605
FDA approves AstraZeneca's anifrolumab for lupus, PMID: 34363027
Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab, PMID: 29420200
Safety profile of anifrolumab in patients with active SLE: an integrated analysis of phase II and III trials, PMID: 33597205
Microglia-dependent synapse loss in type I interferon-mediated lupus, PMID: 28614301
Modulation of Cardiometabolic Disease Markers by Type I Interferon Inhibition in Systemic Lupus Erythematosus, PMID: 32909675
Type I interferon gene signature test-low and -high patients with systemic lupus erythematosus have distinct gene expression signatures, PMID: 31660791
Suppression of T Cell Activation and Collagen Accumulation by an Anti-IFNAR1 mAb, Anifrolumab, in Adult Patients with Systemic Sclerosis, PMID: 25993119
Comment on: 'Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab' by Eric Morand et al, PMID: 30373882
Targeted Biologic Therapy for Systemic Lupus Erythematosus: Emerging Pathways and Drug Pipeline, PMID: 32002918
Response to: 'Comment on: 'Lupus Low Disease Activity State(LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hocanalysis of the Phase IIb MUSE trial of anifrolumab' by Eric Morand et al' by Isenberg, PMID: 30373881
Correction: Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE, PMID: 30613421
A Tale of Two Trials, PMID: 32182394
Anti-IFNαR Mabs for the treatment of systemic lupus erythematosus, PMID: 33085537
String of successful trials in SLE: have we cracked the code?, PMID: 32095250
Exposure-Response Analysis for Selection of Optimal Dosage Regimen of Anifrolumab in Patients With Systemic Lupus Erythematosus, PMID: 33629110
[Biologicals and small molecules for systemic lupus erythematosus], PMID: 32206866
Targeting interferons as a strategy for systemic sclerosis treatment, PMID: 29106987
Type I interferon antagonists in clinical development for lupus, PMID: 32700979
Interferon-targeted therapy in systemic lupus erythematosus: Is this an alternative to targeting B and T cells?, PMID: 27497254
A Successful Trial for Lupus - How Good Is Good Enough?, PMID: 31851796
[New treatments for cutaneous lupus]., PMID:40476397
Use of anifrolumab in a patient with chronic multirefractory Rowell's syndrome., PMID:40450451
Anifrolumab for refractory dermatomyositis: a case series., PMID:40454688
[[Translated article]]Real-World Experience with Anifrolumab in Cutaneous Lupus Erythematosus: Data from a Spanish Tertiary Referral Center., PMID:40446919
Disease Activity-Dependent Siglec-1 Expression on Monocyte Subsets of Patients with Idiopathic Inflammatory Myopathies., PMID:40430089
ERN ReCONNET-SLICC-SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations., PMID:40418946
Potential future biologic therapies for the treatment of vitiligo: focus on phase 2 and 3., PMID:40417830
Rapid response to anifrolumab in refractory systemic lupus erythematosus., PMID:40413848
Cutaneous lupus features specialized stromal niches and altered retroelement expression., PMID:40409678
Atypical infections during combination therapy with anifrolumab and other immunosuppressives in patients with lupus erythematosus: A case series., PMID:40405662
Anifrolumab for refractory discoid lupus: Two case reports of successful outcomes in Saudi Arabia., PMID:40388750
SLE-DAS enables an accurate definition of severe lupus disease activity: derivation and validation in a post hoc study of anifrolumab phase II and III studies., PMID:40379439
Progressive repigmentation of hypopigmented lesions in discoid lupus erythematosus with anifrolumab: A report of two cases., PMID:40378274
An Integrative Mechanistic Model of Type 1 IFN-Mediated Inflammation in Systemic Lupus Erythematosus., PMID:40364448
Adjunctive anifrolumab for recalcitrant discoid lupus erythematosus in the setting of systemic lupus erythematosus: A case report., PMID:40353109
Long-term effect of anifrolumab on patient-reported outcomes in systemic lupus erythematosus (TULIP-LTE): a randomised, placebo-controlled, phase 3 long-term extension trial., PMID:40324450
Combination Therapy With Voclosporin and Anifrolumab in Two Patients With Lupus Nephritis and Discoid Lupus Erythematosus., PMID:40322600
Anifrolumab in childhood-onset systemic lupus erythematosus: a promising option after belimumab failure., PMID:40312207
Anifrolumab in Refractory Dermatomyositis and Antisynthetase Syndrome., PMID:40297385
Hypertrophic lupus erythematosus hypertrophic lichen planus overlap responding to acitretin and anifrolumab., PMID:40276726
Anifrolumab in the management of lupus headaches: a case report., PMID:40242902
A novel treatment for refractory dermatomyositis: A systematic review of anifrolumab and dermatomyositis., PMID:40228662
Development of Diffuse Large B Cell Lymphoma in a Patient with Systemic Lupus Erythematosus within Two Years after the Initiation of Anifrolumab and Mycophenolate Mofetil Treatment., PMID:40222946
Rapid clinical and transcriptomic response to anifrolumab in refractory anti-TIF1γ-positive juvenile dermatomyositis., PMID:40221262
Flare of bullous lupus erythematosus in patients treated with anifrolumab for discoid lupus erythematosus: 2 cases., PMID:40206105
Drug-induced herpes zoster: a pharmacovigilance analysis of FDA adverse event reports from 2004 to 2024., PMID:40206093
RF - New Treatments in Cutaneous Lupus Erythematosus: Current and Future Perspectives., PMID:40204141
Bone marrow MxA staining reflects anifrolumab response in formerly treatment-refractory systemic lupus erythematosus with initial pancytopenia., PMID:40168663
Efficacy of anifrolumab as a first-line therapy for the treatment of thrombocytopenia in systemic lupus erythematosus: a case report., PMID:40153335
Anifrolumab for refractory cutaneous lupus lesions in pediatric systemic lupus erythematosus., PMID:40123095
Comparative efficacy and safety of anifrolumab for belimumab-experienced and biologic-naïve patients with systemic lupus erythematosus: Results from the Kyushu Collagen Disease Network for Systemic Lupus Erythematosus (KCDN-SLE) registry., PMID:40090613
Real-World Experience with Anifrolumab in Cutaneous Lupus Erythematosus: Data from a Spanish Tertiary Referral Center., PMID:40081476
[Translated article] Anifrolumab for the Treatment of Refractory Cutaneous Lupus Erythematosus: A Spanish Clinical Practice-Based Multicenter Study., PMID:40081475
Can Dermoscopy Be a Useful Follow-Up Tool in Patients with Discoid Lupus Treated with Anifrolumab?, PMID:40075770
Systemic lupus erythematosus: one year in review 2025., PMID:40072872
Efficacy and safety profile of anifrolumab in skin lesions of systemic lupus erythematosus., PMID:40052237
A focused report on IFN-1 targeted therapies for lupus erythematosus., PMID:40047795
Improvement in dermatomyositis-associated muscle disease with anifrolumab., PMID:40036364
Sustained Interferon Signature Suppression With Anifrolumab in a Patient With STING-Associated Vasculopathy with Onset in Infancy Refractory to JAK Inhibitor and Dazukibart Therapy., PMID:40007227
Current use and development of monoclonal antibodies for the treatment of systemic lupus erythematosus: a review., PMID:39958566
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials., PMID:39948001
Advances in Targeted Therapy for Systemic Lupus Erythematosus: Current Treatments and Novel Approaches., PMID:39940698
Characteristics and clinical outcomes of patients with systemic lupus erythematosus initiating anifrolumab in a real-world setting in Spain (AZAHAR study): an observational study protocol., PMID:39939126
Successful Treatment of Refractory Cutaneous Dermatomyositis With Anifrolumab., PMID:39925330
Characterization of T-bet expressing B cells in lupus patients indicates a putative prognostic and therapeutic value of these cells for the disease., PMID:39918986
Systemic lupus erythematosus-related macrophage activation syndrome inducing severe cytopenia successfully treated with anifrolumab: a case report., PMID:39907615
Correction to: Anifrolumab in Monogenic Lupus caused by TREX1 Mutation., PMID:39899156
Reduced organ damage accumulation in adult patients with SLE on anifrolumab plus standard of care compared to real-world external controls., PMID:39894690
Interferon activation in bone marrow long-lived plasma cells in systemic lupus erythematosus., PMID:39867907
Systemic Lupus Erythematous: Gene Polymorphisms, Epigenetics, Environmental, Hormonal and Nutritional Factors in the Consideration of Personalized Therapy., PMID:39866364