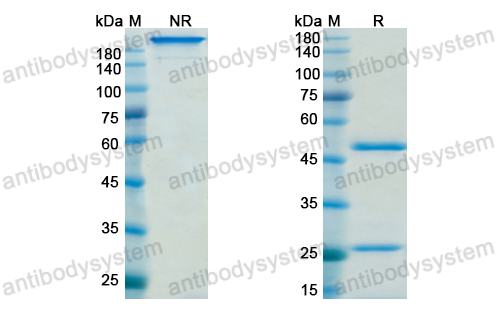
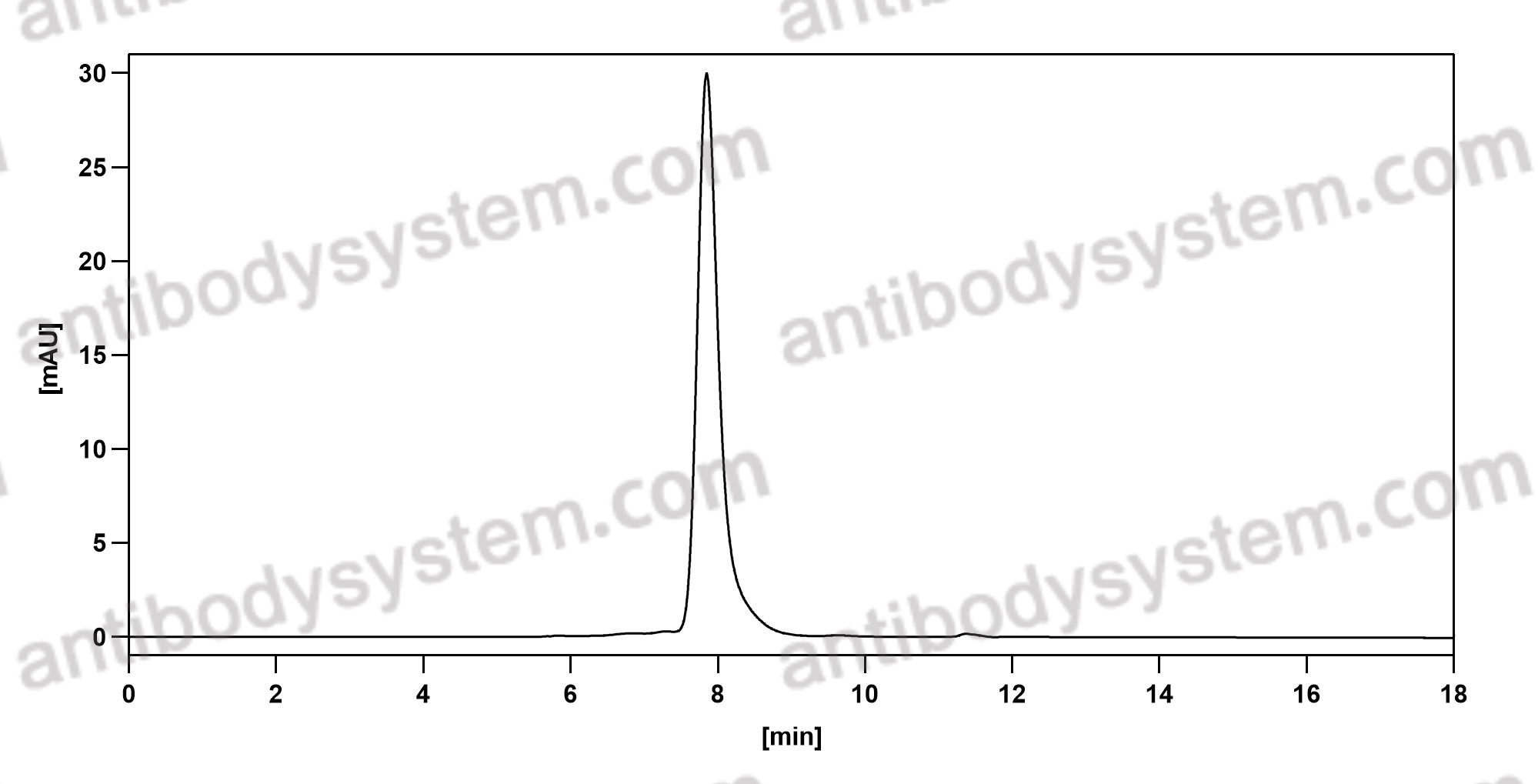
Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus, PMID: 32640132
Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis, PMID: 32074418
Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus, PMID: 31449914
Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study, PMID: 29753033
New and Emerging Systemic Treatments for Atopic Dermatitis, PMID: 32519223
Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis, PMID: 28249150
What's New in Atopic Dermatitis, PMID: 30850043
Biological therapies for atopic dermatitis: An update, PMID: 30679974
Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis, PMID: 29524262
Efficacy of biologics in atopic dermatitis, PMID: 32003247
Drugs for atopic dermatitis, PMID: 32555122
Biologics for Atopic Dermatitis, PMID: 33012322
Nemolizumab in moderate to severe atopic dermatitis: An exploratory analysis of work productivity and activity impairment in a randomized phase II study, PMID: 31166620
New and Emerging Therapies for Pediatric Atopic Dermatitis, PMID: 31364023
Are Biologics Efficacious in Atopic Dermatitis? A Systematic Review and Meta-Analysis, PMID: 29098604
Probiotics for treating eczema, PMID: 30480774
Role of the Pruritic Cytokine IL-31 in Autoimmune Skin Diseases, PMID: 31281316
Emerging Treatment Options in Atopic Dermatitis: Systemic Therapies, PMID: 29320765
Dosage Optimization of Nemolizumab Using Population Pharmacokinetic and Pharmacokinetic-Pharmacodynamic Modeling and Simulation, PMID: 28703903
Current and emerging biologics for the treatment of pediatric atopic dermatitis, PMID: 33078990
Nemolizumab is associated with a rapid improvement in atopic dermatitis signs and symptoms: subpopulation (EASI ≥ 16) analysis of randomized phase 2B study, PMID: 33711179
Efficacy and safety of nemolizumab for adult atopic dermatitis treatment: A meta-analysis of randomized clinical trials, PMID: 33470215
Novel Therapeutic Approaches and Targets for the Treatment of Atopic Dermatitis, PMID: 32525769
Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: a phase II, randomized, double-blind, placebo-controlled clinical study, PMID: 33754202
[No title available], PMID: 30787245
Breaking the Itch-Scratch Cycle in Prurigo Nodularis, PMID: 32074425
Efficacy and Safety of Nemolizumab for Treatment of Adult Atopic Dermatitis, PMID: 34213421
Emerging systemic therapies for atopic dermatitis: biologics, PMID: 33045848
Efficacy and Safety of Nemolizumab for Treatment of Adult Atopic Dermatitis: A Meta-analysis of Randomized Clinical Trials, PMID: 33876738
Itch in Atopic Dermatitis Management, PMID: 27578076
Itch Management: Treatments under Development, PMID: 27578074
Review and analysis of biologic therapies currently in phase II and phase III clinical trials for atopic dermatitis, PMID: 32507066
Novel drugs for the treatment of chronic pruritus, PMID: 30426802
Time to meaningful clinical response in reduction of itch in atopic dermatitis, PMID: 33292019
[New aspects in systemic treatment of atopic dermatitis], PMID: 29470609
[Current and upcoming treatments of adult atopic dermatitis], PMID: 29433635
Interleukin-31 and Pruritic Skin, PMID: 33924978
Recent advances in atopic dermatitis, PMID: 29273133
Interleukin-31 pathway and its role in atopic dermatitis: a systematic review, PMID: 28145790
Therapy for pruritus in the elderly: a review of treatment developments, PMID: 29493371
Biological Therapies for Atopic Dermatitis: A Systematic Review, PMID: 33735876
Novel systemic drugs in treatment of atopic dermatitis: results from phase II and phase III studies published in 2017/2018, PMID: 30095475
Chronic Pruritus: A Review of Neurophysiology and Associated Immune Neuromodulatory Treatments, PMID: 30248162
[What's new in dermatological treatment?], PMID: 29249252
Prurigo Nodularis: Review and Emerging Treatments, PMID: 34077168
The use of biologics in food allergy, PMID: 33966304
Interleukin-31 as a Clinical Target for Pruritus Treatment, PMID: 33644103
New and developing therapies for atopic dermatitis, PMID: 29641707
Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect, PMID: 33685604
What progress have we made in the treatment of atopic eczema? Putting the new biological therapies into a wider context, PMID: 28731235
Four Cases of Prurigo Nodularis Requiring Nemolizumab due to Persistent Pruritus or Lesions after Dupilumab Treatment., PMID:40524402
Effectiveness of Nemolizumab for Refractory Pruritus in a Pediatric Patient With Atopic Dermatitis After Biliary Atresia Surgery., PMID:40491654
Generalized prurigo nodularis after dermatomal herpes zoster: Resolution with nemolizumab., PMID:40491521
Nemolizumab for Cholestatic Pruritus in a Patient With Liver Transplant and Primary Sclerosing Cholangitis., PMID:40465285
Effectiveness of Nemolizumab in Improving Dialysis-Associated Pruritus Refractory to Difelikefalin: A Case Report., PMID:40458322
Maculopapular drug eruption induced by nemolizumab with elevated periostin levels., PMID:40417906
A paediatric case of severe atopic dermatitis with prurigo nodularis successfully treated with nemolizumab as second-line therapy., PMID:40417894
Use of Nemolizumab in the Treatment of Prurigo Nodularis and Atopic Dermatitis., PMID:40403168
Emerging Therapies in the Treatment of Prurigo Nodularis: Biological Therapy and Systematic Review of Literature., PMID:40372666
Nemolizumab Improves Pruritus in Patients With Intrinsic Atopic Dermatitis Lacking Atopic Predisposition: A Single-Centre Pilot Retrospective Cohort Study., PMID:40372094
Deciding Which Patients With Atopic Dermatitis to Prioritize for Biologics and Janus Kinase Inhibitors., PMID:40368249
Cutaneous Adverse Events Following Nemolizumab Administration: A Review., PMID:40364058
Patients' Experiences of Atopic Dermatitis and Nemolizumab Treatment: An In-Trial Interview Study Embedded in a Phase 3 Clinical Trial (ARCADIA)., PMID:40360966
Living network meta-analysis to compare nemolizumab against other available targeted systemic treatments for atopic dermatitis., PMID:40334148
European Guideline (EuroGuiDerm) on atopic eczema: Living update., PMID:40317496
Nemolizumab as a Novel Therapeutic Option for Refractory Prurigo Chronica Multiformis: A Case Report., PMID:40304252
A Case of Cutaneous Fungal Infection Following the Administration of Dupilumab., PMID:40291282
New and Emerging Biologics and Jak Inhibitors for the Treatment of Prurigo Nodularis: A Narrative Review., PMID:40282922
Diffusion dimensionality modeling of subcutaneous/intramuscular absorption of antibodies and long-acting injectables., PMID:40263185
Clinical factors predicting nemolizumab response in atopic dermatitis., PMID:40242149
[Chronic Pruritus: A Review of Its Pathophysiology and Current Treatments]., PMID:40214004
Effectiveness and Safety of Nemolizumab in Patients with Prurigo Nodularis: a Systematic Review and Meta-Analysis of Randomized Controlled Trials., PMID:40205064
Rapid clinical response to nemolizumab in dermatologic diseases associated with pruritus and burning: A multicenter case series., PMID:40122213
Psychometric Validation of the Subject Sleep Diary in Patients with Moderate-to-Severe Atopic Dermatitis., PMID:40116983
Long-term (68 weeks) administration of nemolizumab and topical corticosteroids for prurigo nodularis in patients aged ≥13 years: efficacy and safety data from a phase II/III study., PMID:40112876
A Case of Erythema Multiforme Following the Administration of Nemolizumab., PMID:40104458
Efficacy and Safety of Nemolizumab in Patients With Prurigo Nodularis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials., PMID:40070611
Breaking the Itch Cycle: Long-Term effects of Nemolizumab in Prurigo Nodularis., PMID:40067834
Emerging treatments for dermatologic diseases in infants, children, and adolescents: a systematic review of clinical trials on biologics and small molecule inhibitors., PMID:40042725
Severe Atopic Dermatitis With Increasing Daytime Sleepiness Following Nemolizumab Administration: A Case Report., PMID:40026924
Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2024., PMID:39986987
Immediate and sustained efficacy of nemolizumab in chronic pruritus of unknown origin., PMID:39985241
Comparison chart: Interleukin (IL) receptor antagonists for atopic dermatitis., PMID:39946701
Nemolizumab (Nemluvio) for atopic dermatitis., PMID:39946696
Pre- and Postnatal Development Study of Nemolizumab, a Humanized Anti-Interleukin-31 Receptor A Monoclonal Antibody, in Cynomolgus Monkey., PMID:39868832
Characteristics of suitable cases for treatment with nemolizumab based on clinical findings and cutaneous adverse events., PMID:39817474
English version of clinical practice guidelines for the management of atopic dermatitis 2024., PMID:39707640
Nemolizumab-induced exacerbation of atopic dermatitis., PMID:39698767
Long-term observation to determine durable efficacy and safety of novel paediatric atopic dermatitis treatment, nemolizumab., PMID:39658323
Long-term (68 weeks) administration of nemolizumab in paediatric patients aged 6-12 years with atopic dermatitis with moderate-to-severe pruritus: efficacy and safety data from a phase III study., PMID:39607831
Efficacy and Safety of Nemolizumab in Patients With Moderate to Severe Prurigo Nodularis: The OLYMPIA 1 Randomized Clinical Phase 3 Trial., PMID:39602139
Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review., PMID:39598368
ARCADIA trials: Can nemolizumab be a valid alternative to the therapies currently available?, PMID:39520977
A case of bullous pemphigoid after nemolizumab administration., PMID:39470299
Current and Emerging Biologics for Atopic Dermatitis., PMID:39389711
Correction to: Efficacy and safety of nemolizumab and topical corticosteroids for prurigo nodularis: results from a randomized double-blind placebo-controlled phase II/III clinical study in patients aged ≥ 13 years., PMID:39226086
Bullous Pemphigoid that Developed During Nemolizumab Treatment for Atopic Dermatitis: Two Case Reports., PMID:39221836
Biologic and Small Molecule Therapy in Atopic Dermatitis., PMID:39200305
The role of nemolizumab in the treatment of atopic dermatitis for the adult population., PMID:39119679