Virus-like particle vaccines targeting a key epitope in circumsporozoite protein provide sterilizing immunity against malaria., PMID:40463147
Generation of a Transgenic Plasmodium cynomolgi Parasite Expressing Plasmodium vivax Circumsporozoite Protein for Testing P. vivax CSP-Based Malaria Vaccines in Non-Human Primates., PMID:40432145
Epitope specificity of antibody-mediated protection induced in mice by the malaria vaccine RTS,S/AS01., PMID:40394009
Evidence for a model of conformational change by the Plasmodium falciparum circumsporozoite protein during sporozoite development in the mosquito host through the use of camelid single-domain antibodies., PMID:40293231
Magnitude and durability of ProC6C-AlOH/Matrix-Mtm vaccine-induced malaria transmission-blocking antibodies in Burkinabe adults from a Phase 1 randomized trial., PMID:40208198
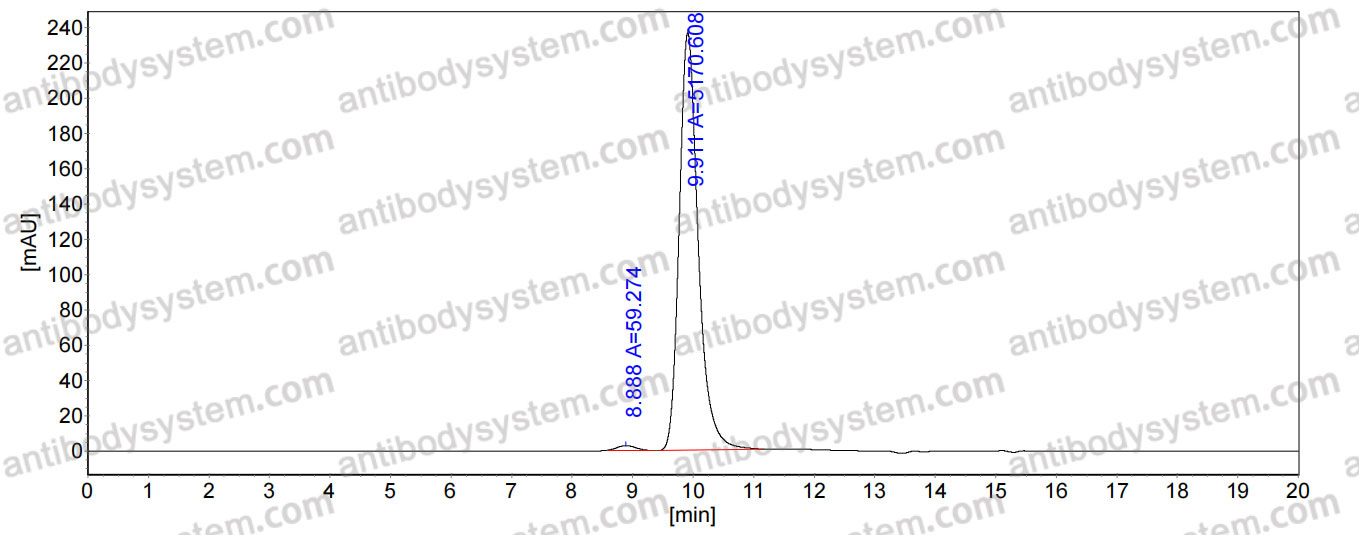
Novel, fully human, anti-PfCSP antibodies with potent antimalarial activity using a phage display based strategy., PMID:40101454
Antibody correlates of risk of clinical malaria in an area of low and unstable malaria transmission in western Kenya., PMID:40033373
iDC-targeting PfCSP mRNA vaccine confers superior protection against Plasmodium compared to conventional mRNA., PMID:39971939
The anti-circumsporozoite antibody response to repeated, seasonal booster doses of the malaria vaccine RTS,S/AS01E., PMID:39915506
Targeting Bottlenecks in Malaria Transmission: Antibody-Epitope Descriptions Guide the Design of Next-Generation Biomedical Interventions., PMID:39907429
PfCSP-ferritin nanoparticle malaria vaccine antigen formulated with aluminum-salt and CpG 1018® adjuvants: Preformulation characterization, antigen-adjuvant interactions, and mouse immunogenicity studies., PMID:39903060
Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein., PMID:39745947
Principal component analysis of the Serological response to Plasmodium Falciparum using a Multiplex bead-based assay in Nigeria., PMID:39730380
Probing novel epitopes on the Plasmodium falciparum circumsporozoite protein for vaccine development., PMID:39557901
ProC6C, a novel multi-stage malaria vaccine, elicits functional antibodies against the minor and central repeats of the Circumsporozoite Protein in human adults., PMID:39555079
The effect of Plasmodium falciparum exposure and maternal anti-circumsporozoite protein antibodies on responses to RTS,S/AS01E vaccination in infants and children: an ancillary observational immunological study to a phase 3, randomised clinical trial., PMID:39461358
Antibody mechanisms of protection against malaria in RTS,S-vaccinated children: a post-hoc serological analysis of phase 2 trial., PMID:39127054
Diversity and selection analyses identify transmission-blocking antigens as the optimal vaccine candidates in Plasmodium falciparum., PMID:39018754
Repeat controlled human Plasmodium falciparum infections delay bloodstream patency and reduce symptoms., PMID:38890271
Plasmodium falciparum AMA1 and CSP antigen diversity in parasite isolates from southern Ghana., PMID:38808064
Diversity and selection analyses identify transmission-blocking antigens as the optimal vaccine candidates in Plasmodium falciparum., PMID:38766239
Innate immune activation restricts priming and protective efficacy of the radiation-attenuated PfSPZ malaria vaccine., PMID:38687615
Vaccines and monoclonal antibodies: new tools for malaria control., PMID:38656211
Antibody response to malaria vaccine candidates in pregnant women with Plasmodium falciparum and Schistosoma haematobium infections., PMID:38587985
A phase IIa, randomized, double-blind, safety, immunogenicity and efficacy trial of Plasmodium falciparum vaccine antigens merozoite surface protein 1 and RTS,S formulated with AS02 adjuvant in healthy, malaria-naïve adults., PMID:38584058
Establishing RTS,S/AS01 as a benchmark for comparison to next-generation malaria vaccines in a mouse model., PMID:38341502
Production and Purification of Plasmodium Circumsporozoite Protein in Lactococcus lactis., PMID:38315362
Profiling the antibody response of humans protected by immunization with Plasmodium vivax radiation-attenuated sporozoites., PMID:38307966
A randomized first-in-human phase I trial of differentially adjuvanted Pfs48/45 malaria vaccines in Burkinabé adults., PMID:38290009
Naturally acquired antibodies against Plasmodium vivax pre-erythrocytic stage vaccine antigens inhibit sporozoite invasion of human hepatocytes in vitro., PMID:38218737
Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine., PMID:38200025
A candidate antibody drug for prevention of malaria., PMID:38167935
Novel antibody competition binding assay identifies distinct serological profiles associated with protection., PMID:38152401
Molecular determinants of cross-reactivity and potency by VH3-33 antibodies against the Plasmodium falciparum circumsporozoite protein., PMID:38007690
Comparative analyses of functional antibody-mediated inhibition with anti-circumsporozoite monoclonal antibodies against transgenic Plasmodium berghei., PMID:37936181
Background malaria incidence and parasitemia during the three-dose RTS,S/AS01 vaccination series do not reduce magnitude of antibody response nor efficacy against the first case of malaria., PMID:37872492
Protective antibody threshold of RTS,S/AS01 malaria vaccine correlates antigen and adjuvant dose in mouse model., PMID:37563255
Preliminary studies on the immunogenicity of a prime-and-trap malaria vaccine in nonhuman primates., PMID:37563050
A multivalent Plasmodium falciparum circumsporozoite protein-based nanoparticle malaria vaccine elicits a robust and durable antibody response against the junctional epitope and the major repeats., PMID:37476056
Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine., PMID:37461621
The αTSR Domain of Plasmodium Circumsporozoite Protein Bound Heparan Sulfates and Elicited High Titers of Sporozoite Binding Antibody After Displayed by Nanoparticles., PMID:37312932
Symptomatic malaria enhances protection from reinfection with homologous Plasmodium falciparum parasites., PMID:37307293
Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine., PMID:37292739
Accumulation of Neutrophil Phagocytic Antibody Features Tracks With Naturally Acquired Immunity Against Malaria in Children., PMID:37150885
Molecular and functional properties of human Plasmodium falciparum CSP C-terminus antibodies., PMID:37082831
Induction of antigen specific intrahepatic CD8+ T cell responses by a secreted heat shock protein based gp96-Ig-PfCA malaria vaccine., PMID:37056783
Serological Markers of Exposure to Plasmodium falciparum and Plasmodium vivax Infection in Southwestern Ethiopia., PMID:37037443
Glycosylated nanoparticle-based PfCSP vaccine confers long-lasting antibody responses and sterile protection in mouse malaria model., PMID:37029167
Evidence of Transmission of Plasmodium vivax 210 and Plasmodium vivax 247 by Anopheles gambiae and An. coluzzii, Major Malaria Vectors in Benin/West Africa., PMID:36975916
Cryo-EM structures of anti-malarial antibody L9 with circumsporozoite protein reveal trimeric L9 association and complete 27-residue epitope., PMID:36931276