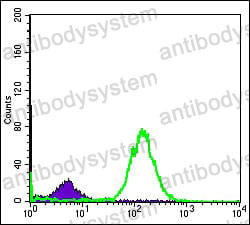
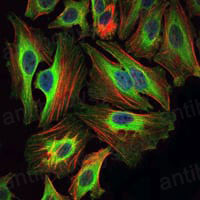
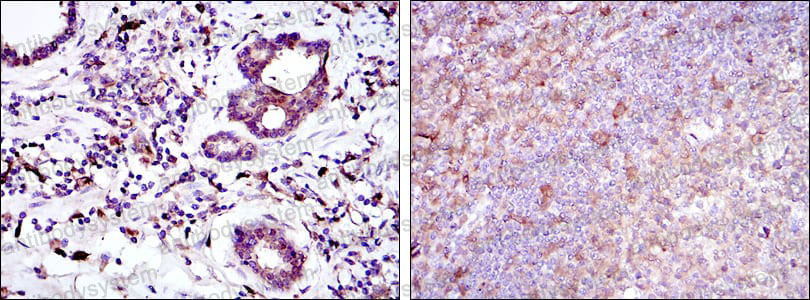
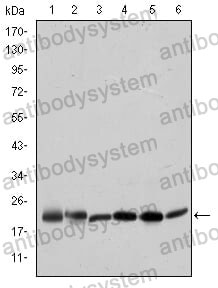
Catalog No.
RHF14802
Species reactivity
Human
Host species
Mouse
Isotype
IgG1
Clonality
Monoclonal
Tested applications
ELISA: 1:10000, FCM: 1:200-1:400, IF: 1:200-1:1000, IHC: 1:200-1:1000, WB: 1:500-1:2000
Target
BH3-interacting domain death agonist, p11 BID, p13 BID, p15 BID, BID, p22 BID
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P55957
Applications
ELISA, FCM, IF, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 0.05% Sodium Azide.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
R3N50
Alectinib in combination with bevacizumab as first-line treatment in ALK-rearranged non-small cell lung cancer (ALEK-B): a single-arm, phase 2 trial., PMID:40379690
Durable responses upon short-term addition of targeted therapy to anti-PD1 in advanced melanoma patients: 5-year progression-free and overall survival update of the IMPemBra trial., PMID:40279684
Comparative efficacy and tolerability of currently approved incretin mimetics: A systematic analysis of placebo-controlled clinical trials., PMID:40212008
Adjuvant TRastuzumab deruxtecan plus fluoropyrimidine versus standard chemotherapy in HER2-positive gastric or gastroesophageal cancer patients with persistence of minimal residual disease in liquid biopsy after pre-operative chemotherapy and radical surgery: the multicentre, phase II randomized TRINITY trial., PMID:40200187
Effect of tofacitinib exposure in utero and during breastfeeding on the immune status of exposed child., PMID:40127036
An interleukin-9-ZBTB18 axis promotes germinal center development of memory B cells., PMID:40107273
A Post Hoc Analysis of Older Patients with Metastatic Colorectal Cancer Receiving Oxaliplatin-Based Chemotherapy Plus Bevacizumab: The Randomized Obelics Study., PMID:40089969
A phase I dose-escalation and expansion study of RMX1002, a selective E-type prostanoid receptor 4 antagonist, as monotherapy and in combination with anti-PD-1 antibody in advanced solid tumors., PMID:39976872
ACE-Breast-02: a randomized phase III trial of ARX788 versus lapatinib plus capecitabine for HER2-positive advanced breast cancer., PMID:39956849
HERV-W Env Induces Neuron Pyroptosis via the NLRP3-CASP1-GSDMD Pathway in Recent-Onset Schizophrenia., PMID:39859234
Human neural rosettes secrete bioactive extracellular vesicles enriched in neuronal and glial cellular components., PMID:39814837
A randomized, double-blind, placebo-controlled, multiple dose, parallel study to investigate the effects of a cathepsin S inhibitor in celiac disease., PMID:39739628
Efficacy and safety of tofacitinib in an open-label, long-term extension study in patients with psoriatic arthritis who received adalimumab or tofacitinib in a Phase 3 randomized controlled study: a post hoc analysis., PMID:39702318
Alternating modified CAPOX/CAPIRI plus bevacizumab in untreated unresectable metastatic colorectal cancer: a phase 2 trial., PMID:39658608
SOX combined with tislelizumab and low-dose radiation therapy for the neoadjuvant treatment of locally advanced gastric/gastroesophageal junction adenocarcinoma: study protocol for a prospective, multicenter, single-arm, phase Ib/II clinical trial., PMID:39606219
Open-Label, Multicenter, Phase I Study to Assess Safety and Tolerability of Adavosertib Plus Durvalumab in Patients with Advanced Solid Tumors., PMID:39560862
Charge-guided masking of a membrane-destabilizing peptide enables efficient endosomal escape for targeted intracellular delivery of proteins., PMID:39525569
Forimtamig, a novel GPRC5D-targeting T-cell bispecific antibody with a 2+1 format, for the treatment of multiple myeloma., PMID:39476124
Serum-derived bovine immunoglobulin treatment in COVID-19 is associated with faster resolution of symptoms: A randomized pilot clinical trial., PMID:39390688
Pooled analysis of the MANTICO2 and MONET randomized controlled trials comparing drug efficacy for early treatment of COVID-19 during Omicron waves., PMID:39343244
Efficacy and safety of camrelizumab, apatinib, and capecitabine combination therapy in advanced biliary tract cancer: a phase 2, nonrandomized, prospective study., PMID:39102756
Epacadostat plus pembrolizumab versus placebo plus pembrolizumab for advanced urothelial carcinoma: results from the randomized phase III ECHO-303/KEYNOTE-698 study., PMID:39054485
Pembrolizumab with platinum-based chemotherapy with or without epacadostat as first-line treatment for metastatic non-small cell lung cancer: a randomized, partially double-blind, placebo-controlled phase II study., PMID:39054462
Avacopan for anti-neutrophil cytoplasm antibodies-associated vasculitis: a multicentre real-world study., PMID:39001799
Targeted serum proteome profiling reveals nicotinamide adenine dinucleotide phosphate (NADPH)-related biomarkers to discriminate linear IgA bullous disorder from dermatitis herpetiformis., PMID:38908771
Camrelizumab-based induction chemoimmunotherapy in locally advanced stage hypopharyngeal carcinoma: phase II clinical trial., PMID:38898018
Discovery of a potent, selective, and tumor-suppressing antibody antagonist of adenosine A2A receptor., PMID:38837964
Tofacitinib to prevent anti-drug antibody formation against LMB-100 immunotoxin in patients with advanced mesothelin-expressing cancers., PMID:38706610
PD-1 inhibition with retifanlimab and/or arginase inhibition with INCB001158 in Japanese patients with solid tumors: A phase I study., PMID:38651187
Derazantinib alone and with atezolizumab in metastatic urothelial carcinoma with activating FGFR aberrations., PMID:38627238
Binimetinib in combination with nivolumab or nivolumab and ipilimumab in patients with previously treated microsatellite-stable metastatic colorectal cancer with RAS mutations in an open-label phase 1b/2 study., PMID:38600471
Efficacy and safety of second-line therapy by S-1 combined with sintilimab and anlotinib in pancreatic cancer patients with liver metastasis: a single-arm, phase II clinical trial., PMID:38361920
Compatible co-administration of BioThrax® vaccine and ciprofloxacin-Results of a randomized open-label drug-vaccine interaction trial., PMID:38352727
Successful xenotransplantation of testicular cells following fractionated chemotherapy of recipient birds., PMID:38321093
Phase I study of peposertib and avelumab with or without palliative radiotherapy in patients with advanced solid tumors., PMID:38320431
Novel chimeric antigen receptor-expressing T cells targeting the malignant mesothelioma-specific antigen sialylated HEG1., PMID:38212893
A Successfully Treated COVID-19 Vaccine Induced Immune Thrombocytopenic Purpura., PMID:38174163
Impaired FADD/BID signaling mediates cross-resistance to immunotherapy in Multiple Myeloma., PMID:38129580
Base-Resolution Sequencing Methods for Whole-Transcriptome Quantification of mRNA Modifications., PMID:38079380
Dermatoscopic Features of Early Erythema Chronicum Migrans., PMID:38006374
Phase II clinical trial of neoadjuvant anti-PD-1 (toripalimab) combined with axitinib in resectable mucosal melanoma., PMID:37956739
TOX, TWIST1, STAT4, and SATB1 protein expressions in early-stage mycosis fungoides., PMID:37932931
Rilzabrutinib versus placebo in adults and adolescents with persistent or chronic immune thrombocytopenia: LUNA 3 phase III study., PMID:37869360
Cost-effectiveness analysis of nivolumab plus chemotherapy vs chemotherapy for patients with unresectable advanced or metastatic HER2-negative gastric or gastroesophageal junction or esophageal adenocarcinoma in Japan., PMID:37725256
Hypertrophic obstructive cardiomyopathy caused by Fabry disease: implications for surgical myectomy., PMID:37715354
Mytomicin-C, Metronomic Capecitabine, and Bevacizumab in Patients With Unresectable or Relapsed Pseudomyxoma Peritonei of Appendiceal Origin., PMID:37657955
Rare combination of chronic primary adrenal insufficiency, subclinical hypothyroidism, and bicytopenia as features of systemic lupus erythematosus in a young man: A case report., PMID:37539357
Ruxolitinib versus basiliximab for steroid-refractory acute graft-versus-host disease: a retrospective study., PMID:37474631
Ramucirumab beyond progression plus TAS-102 in patients with advanced or metastatic esophagogastric adenocarcinoma, after treatment failure on a ramucirumab-based therapy., PMID:37455496
Preclinical and clinical activity of DZD1516, a full blood-brain barrier-penetrant, highly selective HER2 inhibitor., PMID:37415239