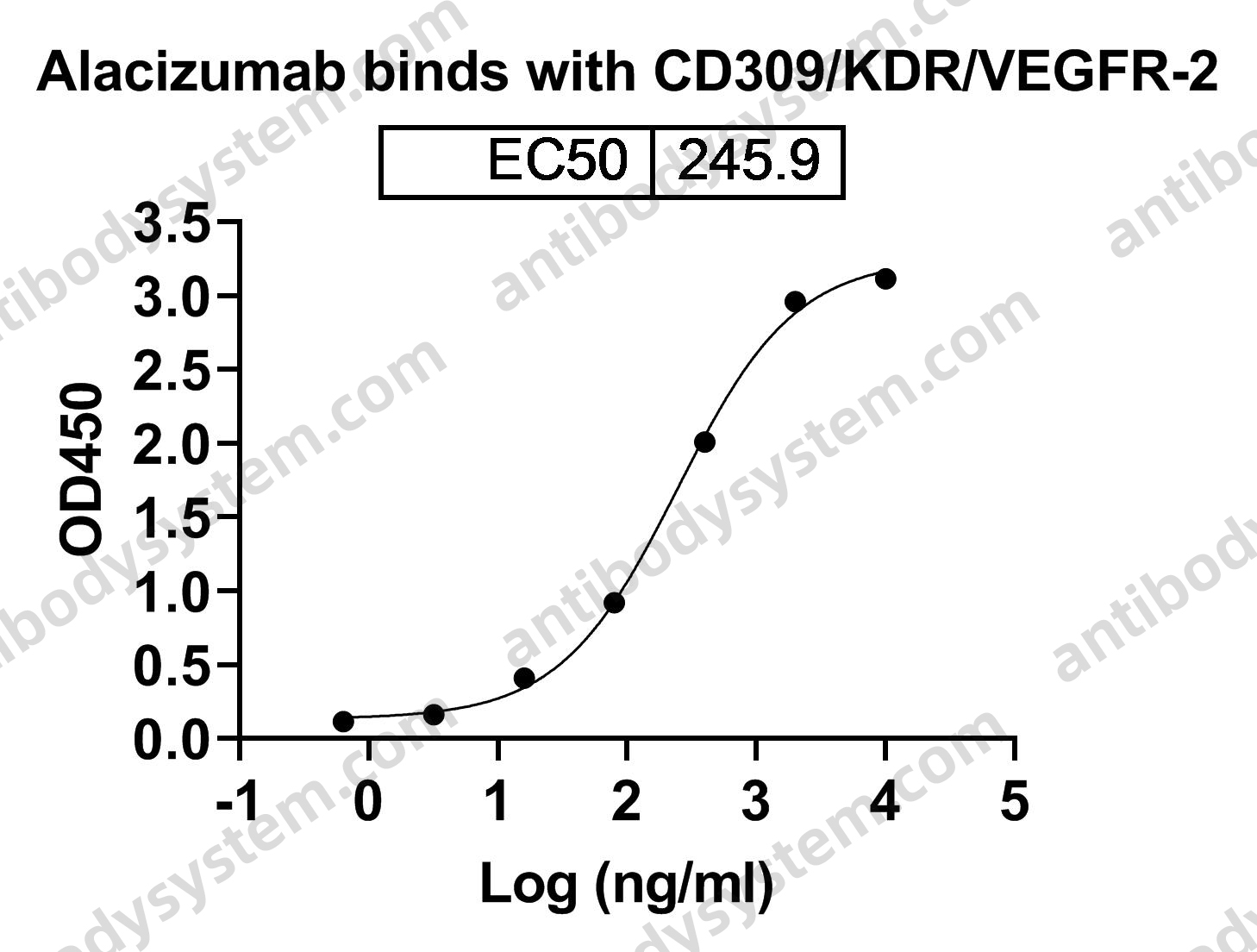
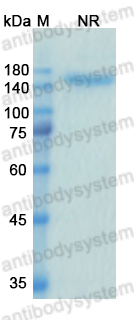
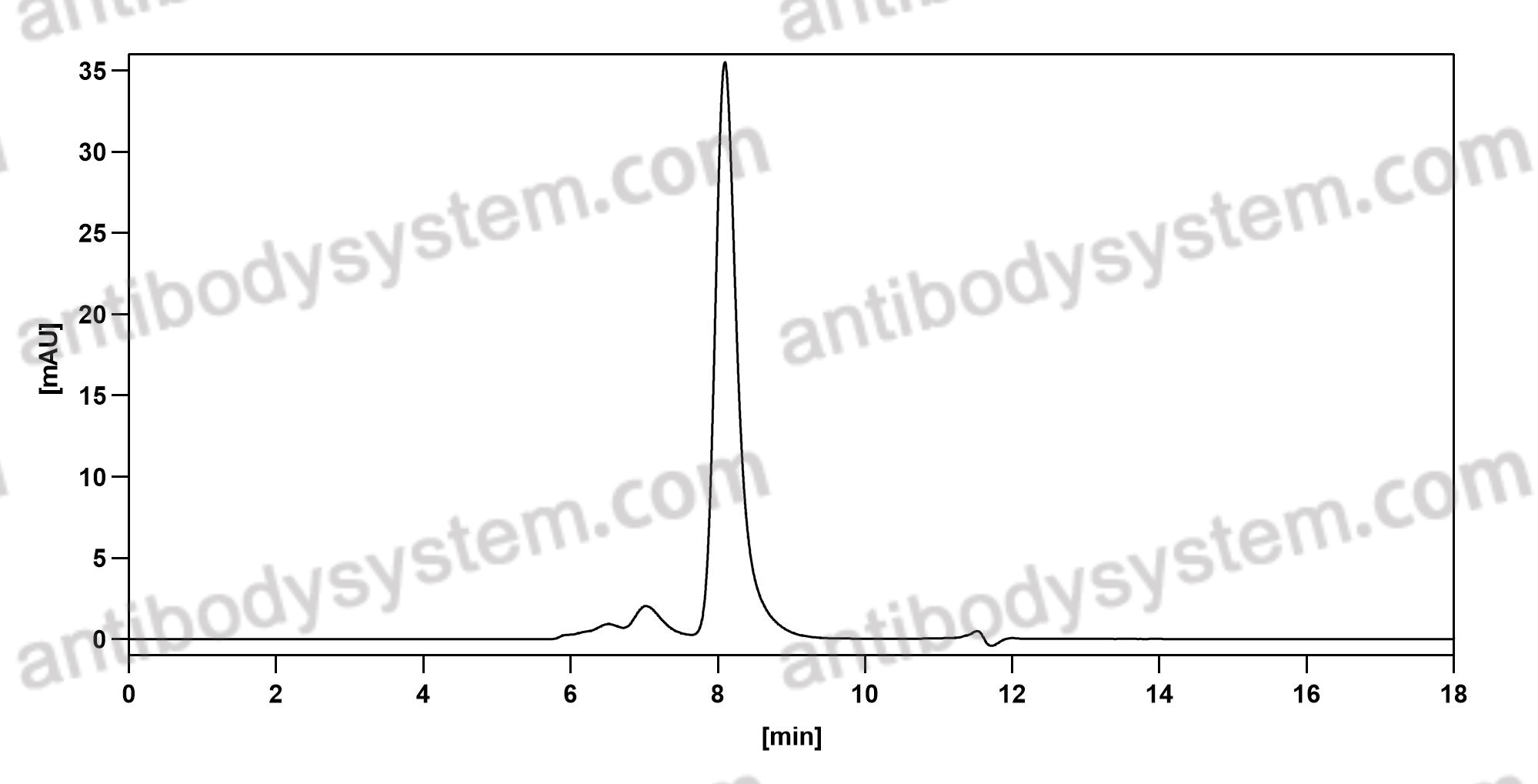
Catalog No.
DHE16603
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG4
Clonality
Monoclonal
Target
FLK-1, Kinase insert domain receptor, VEGFR2, Fetal liver kinase 1, CD309, Vascular endothelial growth factor receptor 2, Protein-tyrosine kinase receptor flk-1, FLK1, VEGFR-2, KDR
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P35968
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
Alacizumab
Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 30625070
Caplacizumab: First Global Approval, PMID: 30298461
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 26863353
Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura, PMID: 32095224
Caplacizumab: an anti-von Willebrand factor antibody for the treatment of thrombotic thrombocytopenic purpura, PMID: 32588878
Caplacizumab: a change in the paradigm of thrombotic thrombocytopenic purpura treatment, PMID: 31359806
A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP, PMID: 33150928
Caplacizumab to treat immune-mediated thrombotic thrombocytopenic purpura, PMID: 31250841
Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura, PMID: 33280030
Real-world experience with caplacizumab in the management of acute TTP, PMID: 33150355
Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura, PMID: 32634236
Efficacy and safety of open-label caplacizumab in patients with exacerbations of acquired thrombotic thrombocytopenic purpura in the HERCULES study, PMID: 31691462
Caplacizumab, PMID: 30933455
ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP, PMID: 32634237
Clinical pharmacology of caplacizumab for the treatment of patients with acquired thrombotic thrombocytopenic purpura, PMID: 30977686
A critical evaluation of caplacizumab for the treatment of acquired thrombotic thrombocytopenic purpura, PMID: 32876503
Should all patients with immune-mediated thrombotic thrombocytopenic purpura receive caplacizumab?, PMID: 33236389
Caplacizumab (Cablivi) for iTTP, PMID: 33429409
Caplacizumab as an emerging treatment option for acquired thrombotic thrombocytopenic purpura, PMID: 31118566
Caplacizumab: frequent local skin reactions, PMID: 32997190
Caplacizumab for relapsing thrombotic thrombocytopenic purpura, PMID: 31177334
Thrombotic thrombocytopenic purpura, PMID: 28416507
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31042846
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31042845
Caplacizumab for thrombotic thrombocytopenic purpura, PMID: 33664548
The Therapeutic Potential of Nanobodies, PMID: 31686399
Caplacizumab, PMID: 34251781
Caplacizumab for treatment of thrombotic thrombocytopenic purpura in a patient with anaphylaxis to fresh-frozen plasma, PMID: 32358818
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. Reply, PMID: 31042847
Clinical Efficacy and Safety Profile of Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31576256
Caplacizumab treatment for acquired refractory thrombotic thrombocytopenic purpura, PMID: 32738052
Refractory Auto-Immune Thrombotic Thrombocytopenic Pupura Successfully Treated With Caplacizumab, PMID: 33195299
Role of caplacizumab in the treatment of acquired thrombotic thrombocytopenic purpura, PMID: 32605495
ISTH guidelines for treatment of thrombotic thrombocytopenic purpura, PMID: 32914526
Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura (HERCULES trial), PMID: 31318128
Pharmacoeconomic Review Report: Caplacizumab (Cablivi): (Sanofi Genzyme, a division of Sanofi-Aventis Canada Inc.) [Internet], PMID: 33570888
New Therapeutic Targets and Treatment Options for Thrombotic Microangiopathy: Caplacizumab and Ravulizumab, PMID: 32715723
Counting the cost of caplacizumab, PMID: 33599760
Antibodies to watch in 2019, PMID: 30516432
Caplacizumab Therapy without Plasma Exchange for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 31269374
Correction to: Caplacizumab: First Global Approval, PMID: 30511322
Successful use of caplacizumab in a case of refractory acquired thrombotic thrombocytopenic purpura following subacute thyroiditis, PMID: 33223471
ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura, PMID: 32914582
Effective and safe off-label use of caplacizumab treatment in a middle-aged obese male, PMID: 33285299
Clinical Review Report: Caplacizumab (Cablivi): (Sanofi Genzyme, a division of Sanofi-Aventis Canada Inc.) [Internet], PMID: 33600100
Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura, PMID: 28445600
Shared decision making, thrombotic thrombocytopenic purpura, and caplacizumab, PMID: 31894592
Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura, PMID: 27332911
Nanobody Engineering: Toward Next Generation Immunotherapies and Immunoimaging of Cancer, PMID: 31544819
Caplacizumab for Acute Thrombotic Thrombocytopenic Purpura, PMID: 34109052