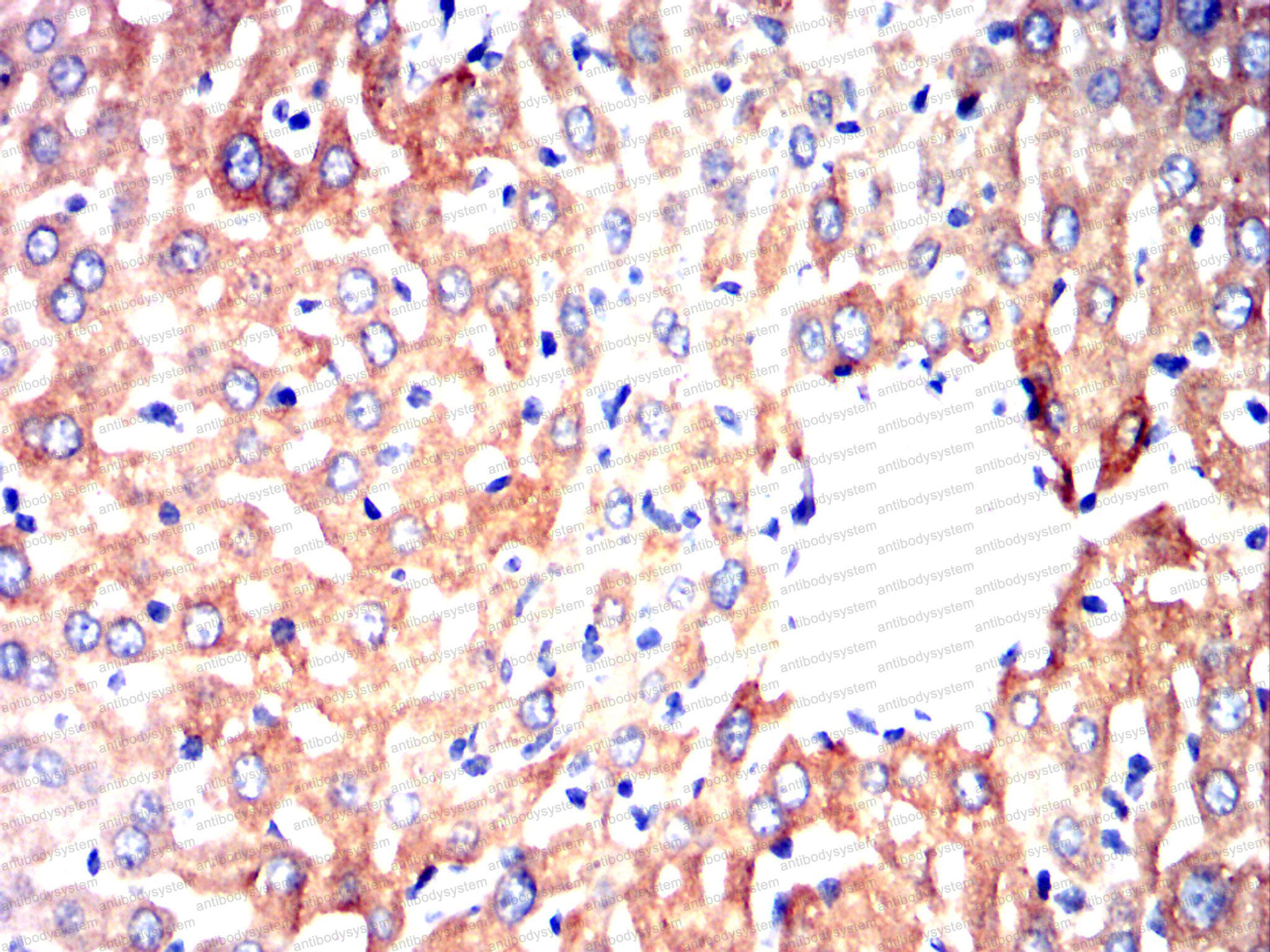
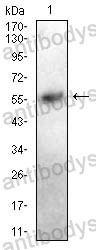
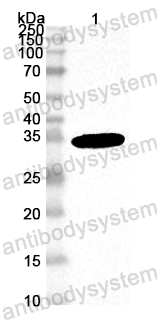
Catalog No.
RHC35402
Species reactivity
Human, Mouse, Rat
Host species
Mouse
Isotype
IgG1
Clonality
Monoclonal
Tested applications
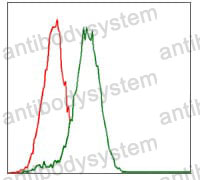
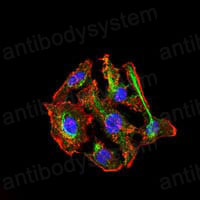
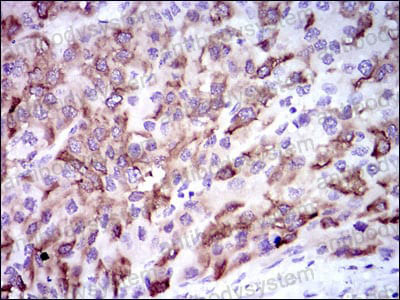
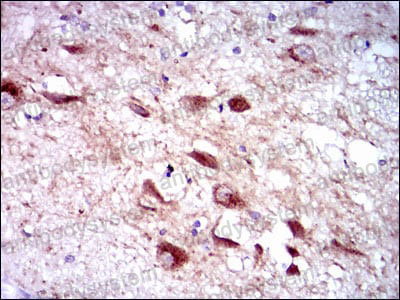
ELISA: 1:10000, FCM: 1:200-1:400, IF: 1:50-1:500, IHC: 1:100-1:500, WB: 1:500-1:2000
Target
CYPIIIA3, Cytochrome P450 3A4, 1,4-cineole 2-exo-monooxygenase, CYP3A4, Cytochrome P450 3A3, Cytochrome P450 NF-25, Cytochrome P450-PCN1, CYPIIIA4, 1,8-cineole 2-exo-monooxygenase, Albendazole monooxygenase (sulfoxide-forming), Nifedipine oxidase, Quinine 3-monooxygenase, CYP3A3, Cholesterol 25-hydroxylase, Cytochrome P450 HLp, Albendazole sulfoxidase
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P08684
Applications
ELISA, FCM, IF, IHC, WB
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4, 0.05% Sodium Azide.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
R3B24
Construction and evaluation of glucocorticoid dose prediction model based on genetic and clinical characteristics of patients with systemic lupus erythematosus., PMID:40186486
Evaluation of the Effect of Risankizumab on the Pharmacokinetics of Cytochrome P450 Substrates in Patients with Moderately to Severely Active Ulcerative Colitis or Crohn's Disease., PMID:39707077
Evaluation of Drug-Drug Interaction Potential of Talquetamab, a T-Cell-Redirecting GPRC5D × CD3 Bispecific Antibody, as a Result of Cytokine Release Syndrome in Patients with Relapsed/Refractory Multiple Myeloma in MonumenTAL-1, Using a Physiologically Based Pharmacokinetic Model., PMID:39285155
Effect of Mild or Moderate Hepatic Impairment on the Pharmacokinetics of Avacopan, a Small-Molecule Complement C5a Receptor Antagonist, for the Treatment of Antineutrophil Cytoplasmic Autoantibody-Associated Vasculitis., PMID:38993026
Inflammation-mediated drug interactions of olokizumab and cytochrome P450 activities in patients with rheumatoid arthritis., PMID:38984761
In Vitro Metabolism and Transport Characteristics of Zastaprazan., PMID:38931920
Tacrolimus personalized therapy based on CYP3A5 genotype in Chinese patients with idiopathic inflammatory myopathies., PMID:38889292
An in silico investigation of the toxicological effects and biological activities of 3-phenoxybenzoic acid and its metabolite products., PMID:38833509
Exploratory associations of tacrolimus exposure and clinical outcomes after lung transplantation: A retrospective, single center experience., PMID:38363388
Immune outcomes of lung transplant recipients with different cytochrome P450 3A5 phenotypes after discontinuation of voriconazole antifungal prophylaxis., PMID:38289893
Assessment of CYP3A-mediated drug interaction via cytokine (IL-6) elevation for mosunetuzumab using physiologically-based pharmacokinetic modeling., PMID:38050329
Evaluation of the potential impact on pharmacokinetics of various cytochrome P450 substrates of increasing IL-6 levels following administration of the T-cell bispecific engager glofitamab., PMID:38044486
Prediction of CYP Down Regulation after Tusamitamab Ravtansine Administration (a DM4-Conjugate), Based on an In Vitro-In Vivo Extrapolation Approach., PMID:37964462
Adverse Event Profile Differences between Trastuzumab Emtansine and Trastuzumab Deruxtecan: A Real-world, Pharmacovigilance Study., PMID:37928419
Metabolic and toxicological considerations regarding CGRP mAbs and CGRP antagonists to treat migraine in COVID-19 patients: a narrative review., PMID:37925645
Population pharmacokinetics of mirvetuximab soravtansine in patients with folate receptor-α positive ovarian cancer: The antibody-drug conjugate, payload and metabolite., PMID:37872122
Possible New Histological Prognostic Index for Large B-Cell Lymphoma., PMID:37834968
Physiologically based pharmacokinetic model to predict drug-drug interactions with the antibody-drug conjugate enfortumab vedotin., PMID:37632598
Brentuximab vedotin with AVD for stage II-IV HIV-related Hodgkin lymphoma (AMC 085): phase 2 results from an open-label, single arm, multicentre phase 1/2 trial., PMID:37532416
The Influence of Lipid Microdomain Heterogeneity on Protein-Protein Interactions: Proteomic Analysis of Co-Immunoprecipitated Binding Partners of P450 1A2 and P450 3A in Rat Liver Microsomes., PMID:37349115
Liver microsomal cytochrome P450 3A-dependent drug oxidation activities in individual dogs., PMID:37144920
Do antibody-drug conjugates increase the risk of sepsis in cancer patients? A pharmacovigilance study., PMID:36467034
Concomitant Use of Ado-trastuzumab Emtansine and Imatinib in a Patient of CML and Metastatic Breast Cancer., PMID:36377026
[Acute Myeloid Leukemia - Update 2022]., PMID:36030783
Dietary aflatoxin B1 caused the growth inhibition, and activated oxidative stress and endoplasmic reticulum stress pathway, inducing apoptosis and inflammation in the liver of northern snakehead (Channa argus)., PMID:35964742
Clinically Relevant Interactions Between Ritonavir-Boosted Nirmatrelvir and Concomitant Antiseizure Medications: Implications for the Management of COVID-19 in Patients with Epilepsy., PMID:35895276
Tacrolimus CYP3A Single-Nucleotide Polymorphisms and Preformed T- and B-Cell Alloimmune Memory Improve Current Pretransplant Rejection-Risk Stratification in Kidney Transplantation., PMID:35833145
Development of a UPLC-MRM-based targeted proteomic method to profile subcellular organelle marker proteins from human liver tissues., PMID:35768540
Effects of tacrolimus intrapatient variability and CYP3A5 polymorphism on the outcomes of pediatric kidney transplantation., PMID:35466485
Mycotoxins binder supplementation alleviates aflatoxin B1 toxic effects on the immune response and intestinal barrier function in broilers., PMID:35121530
Interfering with cholesterol metabolism impairs tick embryo development and turns eggs susceptible to bacterial colonization., PMID:34325088
Development of De Novo Donor-specific HLA Antibodies and AMR in Renal Transplant Patients Depends on CYP3A5 Genotype., PMID:34241984
Anti-interleukin-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection: effect on cytochrome P450 drug metabolism., PMID:34153143
Impact of CYP3A5 phenotype on tacrolimus time in therapeutic range and clinical outcomes in pediatric renal and heart transplant recipients., PMID:34129685
Management of COVID-19 in patients with seizures: Mechanisms of action of potential COVID-19 drug treatments and consideration for potential drug-drug interactions with anti-seizure medications., PMID:34044300
Severe secondary hyperkalemia and arrhythmia from drug interactions between calcium-channel blocker and voriconazole: a case presentation., PMID:33971831
The effect of ivermectin alone and in combination with cobicistat or elacridar in experimental Schistosoma mansoni infection in mice., PMID:33627744
Case Report: Signal Transducer and Activator of Transcription 3 Gain-of-Function and Spectrin Deficiency: A Life-Threatening Case of Severe Hemolytic Anemia., PMID:33519826
Real-world prevalence and consequences of potential drug-drug interactions in the first-wave COVID-19 treatments., PMID:33368439
Assessment of Vedolizumab Disease-Drug-Drug Interaction Potential in Patients With Inflammatory Bowel Diseases., PMID:33331142
Pharmacokinetics, Pharmacodynamics and Drug-Drug Interactions of New Anti-Migraine Drugs-Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies., PMID:33287305
High Intrapatient Variability in Tacrolimus Exposure Calculated Over a Long Period Is Associated With De Novo Donor-Specific Antibody Development and/or Late Rejection in Thai Kidney Transplant Patients Receiving Concomitant CYP3A4/5 Inhibitors., PMID:33278239
Physiologically Based Pharmacokinetic Model-Informed Drug Development for Polatuzumab Vedotin: Label for Drug-Drug Interactions Without Dedicated Clinical Trials., PMID:33205435
Metabolism and Distribution of Novel Tumor Targeting Drugs In Vivo., PMID:33183197
Regioselective Hydroxylation of Phloretin, a Bioactive Compound from Apples, by Human Cytochrome P450 Enzymes., PMID:33105851
Gender differences in plasma pharmacokinetics and hepatic metabolism of geissoschizine methyl ether from Uncaria hook in rats., PMID:32898626
Midazolam oxidation in cattle liver microsomes: The role of cytochrome P450 3A., PMID:32893906
FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy., PMID:32717568
Effect of Systemic Inflammatory Response to SARS-CoV-2 on Lopinavir and Hydroxychloroquine Plasma Concentrations., PMID:32641296
Rhabdomyolysis as an initial presentation in a patient diagnosed with COVID-19., PMID:32587121