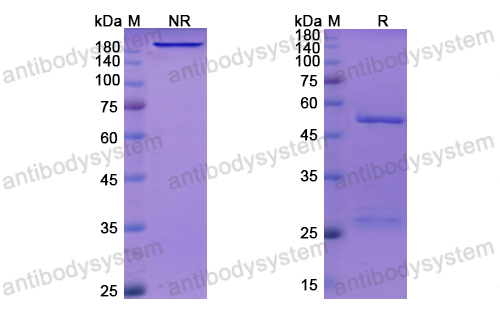
Camrelizumab: First Global Approval, PMID: 31313098
Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study, PMID: 32416073
Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial, PMID: 33347829
Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial, PMID: 32112738
Addition of Low-Dose Decitabine to Anti-PD-1 Antibody Camrelizumab in Relapsed/Refractory Classical Hodgkin Lymphoma, PMID: 31039052
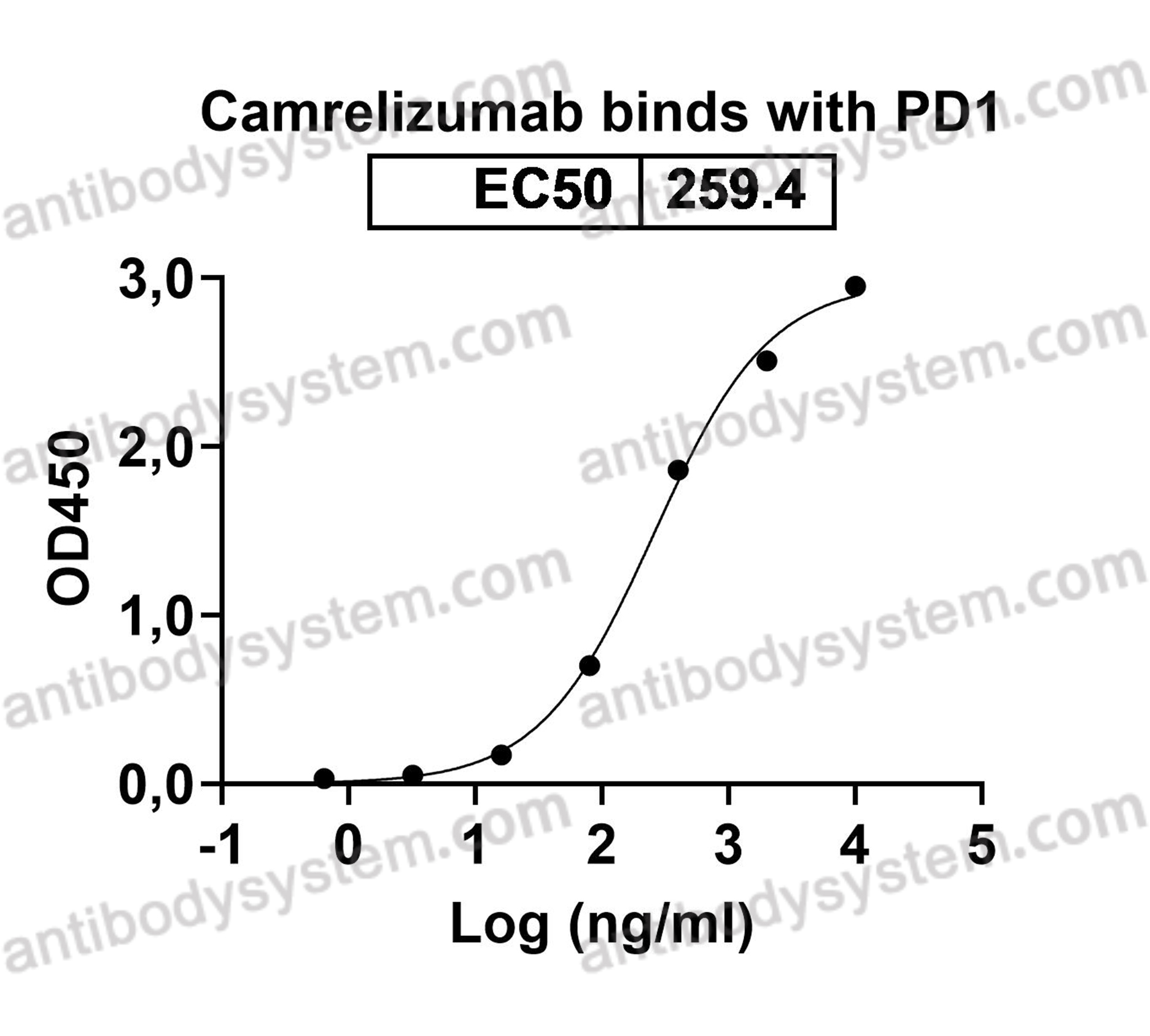
N-glycosylation of PD-1 promotes binding of camrelizumab, PMID: 33063473
The clinical application of camrelizumab on advanced hepatocellular carcinoma, PMID: 32762583
Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial, PMID: 33087333
Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial, PMID: 33166719
Camrelizumab Plus Apatinib in Patients With Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial, PMID: 33052760
Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials, PMID: 30213452
Camrelizumab plus gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer: a single-arm, open-label, phase II trial, PMID: 33172881
Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients, PMID: 33241036
Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial, PMID: 32393323
A Single-Arm, Multicenter, Phase II Study of Camrelizumab in Relapsed or Refractory Classical Hodgkin Lymphoma, PMID: 31420358
Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy, PMID: 33323401
Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma, PMID: 33314747
Efficacy and Safety of SBRT Combined With Camrelizumab and Apatinib in HCC Patients With PVTT: Study Protocol of a Randomized Controlled Trial, PMID: 32984021
Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial, PMID: 32448804
Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial, PMID: 32376724
Camrelizumab-targeting a novel PD-1 epitope to treat hepatocellular carcinoma, PMID: 33437813
Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer, PMID: 31939448
Salvage camrelizumab plus apatinib for relapsed esophageal neuroendocrine carcinoma after esophagectomy: a case report and review of the literature, PMID: 33092443
Camrelizumab for nasopharyngeal carcinoma: a new hope?, PMID: 30213451
Camrelizumab plus apatinib successfully treated a patient with advanced esophageal squamous cell carcinoma, PMID: 32814482
A First-in-Human Dose Finding Study of Camrelizumab in Patients with Advanced or Metastatic Cancer in Australia, PMID: 32256049
Camrelizumab Plus Gemcitabine, Vinorelbine, and Pegylated Liposomal Doxorubicin in Relapsed/Refractory Primary Mediastinal B-Cell Lymphoma: A Single-Arm, Open-Label, Phase II Trial, PMID: 32499235
Correction to: Camrelizumab: First Global Approval, PMID: 31446564
Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: an open-label prospective pivotal trial, PMID: 32623573
Rechallenge of camrelizumab in non-small-cell lung cancer patients treated previously with camrelizumab and microwave ablation, PMID: 33004770
Antibodies to watch in 2019, PMID: 30516432
Experience With Anti-PD-1 Antibody, Camrelizumab, Monotherapy for Biliary Tract Cancer Patients and Literature Review, PMID: 33308041
Camrelizumab (SHR-1210) leading to reactive capillary hemangioma in the gingiva: A case report, PMID: 32110675
Population pharmacokinetics of the anti-PD-1 antibody camrelizumab in patients with multiple tumor types and model-informed dosing strategy, PMID: 33154554
Anti-PD-1 antibody camrelizumab plus doxorubicin showed durable response in pulmonary sarcomatoid carcinoma: Case report and literature review, PMID: 32776600
Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study, PMID: 30348638
Emerging agents and regimens for hepatocellular carcinoma, PMID: 31655607
Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer, PMID: 30755403
Immune-Related Adverse Events Mimicking Behcet's Disease in a Gastric Cancer Patient Following Camrelizumab Treatment, PMID: 32602470
A large randomized clinical trial is necessary to establish the role of camrelizumab in hepatocellular carcinoma, PMID: 33178785
Immune checkpoint inhibitor-related pneumonitis induced by camrelizumab: a case report and review of literature, PMID: 34044548
Comparative safety and efficacy of anti-PD-1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: findings from recent advances in landmark trials, PMID: 31238988
Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma, PMID: 31337439
Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial, PMID: 34174189
Improved clinical outcome in a randomized phase II study of anti-PD-1 camrelizumab plus decitabine in relapsed/refractory Hodgkin lymphoma, PMID: 33820822
Camrelizumab efficacious in NPC, PMID: 34253913
Immune checkpoint inhibitors in advanced nasopharyngeal carcinoma: Beyond an era of chemoradiation?, PMID: 31950498
Camrelizumab plus famitinib to treat RCC, PMID: 34453154
Novel Therapeutics for Recurrent Cervical Cancer: Moving Towards Personalized Therapy, PMID: 31939072
Protocol for a randomized controlled trial of perioperative S-1 plus oxaliplatin combined with apatinib and camrelizumab in patients with resectable, locally advanced gastric or gastroesophageal junction adenocarcinoma, PMID: 33490196
Extended progression-free survival after combination treatment with anlotinib and S-1 in refractory advanced esophageal cancer: A case report., PMID:40529101
Assessing the effectiveness of camrelizumab plus anti-angiogenesis drug for the treatment of advanced liver cancer: a single‑center retrospective study., PMID:40521632
High level of anti-drug antibodies is associated with shorter survival in advanced solid cancer patients treated with Immune checkpoint inhibitors., PMID:40520330
Obesity paradox role in the immunosuppressive treatment of hepatocellular carcinoma., PMID:40497086
An Advanced IVB Lung Adenocarcinoma Patient With KRAS Mutations, Benefited From Camrelizumab Combined With Anti-Angiogenic Agents for Therapy: A Case Report., PMID:40488279
Effectiveness and Safety of TRIPLET Protocol in Patients with Intermediate-Stage Hepatocellular Carcinoma to Transcatheter Arterial Chemoembolization Refractory., PMID:40480939
Efficacy of transarterial chemoembolization-hepatic arterial infusion chemotherapy combined with targeted therapy and immunotherapy in hepatocellular carcinoma with portal vein tumor thrombosis., PMID:40469916
Effectiveness and safety of camrelizumab plus apatinib in treating recurrent/metastatic nasopharyngeal carcinoma: a single-arm meta-analysis., PMID:40450144
Rechallenge of anti-PD-1 antibody combined with chemotherapy shows promising efficacy in the treatment of advanced metastatic hepatocellular carcinoma: A case report., PMID:40438874
Immunotherapy combined with chemotherapy: a promising therapeutic approach in the management of colonic squamous cell carcinoma-a case report., PMID:40416862
The Importance of Timing in Immunotherapy: A Systematic Review., PMID:40416194
SMARCA4-deficient undifferentiated thoracic tumor: a case report and literature review., PMID:40400987
Association between reactive cutaneous capillary endothelial proliferation and the efficacy of camrelizumab in esophageal cancer: a retrospective cohort study., PMID:40400922
Camrelizumab in combination with chemotherapy and targeted therapy improves the prognosis in patients with advanced biliary tract cancer: a single-center retrospective clinical study., PMID:40386592
Efficacy and safety of camrelizumab in combination with S-1 plus oxaliplatin sequenced by camrelizumab-based maintenance therapy as a first-line treatment for advanced gastric or gastroesophageal junction adenocarcinoma: a retrospective cohort study., PMID:40386583
Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for patients with unresectable hepatocellular carcinoma: a cost-utility analysis in China and the United States., PMID:40376272
Haematological toxicities with immune checkpoint inhibitors in digestive system tumors: a systematic review and network meta-analysis of randomized controlled trials., PMID:40360867
Clinical manifestations and risk factors of immune-related thyroid adverse events in patients treated with PD-1 inhibitors: a case-control study., PMID:40356893
Biomarkers of response to camrelizumab combined with apatinib: an analysis from a phase II trial in recurrent/metastatic nasopharyngeal carcinoma., PMID:40355717
Molecular imaging using 18F-FDG PET/CT and circulating inflammatory and immune indicators to predict pathological response to neoadjuvant camrelizumab plus chemotherapy in resectable stage IIIA-IIIB NSCLC., PMID:40348946
Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy for locally advanced rectal cancer: 3-year survival from a phase 2 study., PMID:40346524
Camrelizumab combined with apatinib plus irinotecan as a second-line treatment in advanced or metastatic esophageal squamous cell carcinoma patients., PMID:40346506
Hepatic artery infusion of FOLFOX chemotherapy plus camrelizumab combined with sorafenib for advanced hepatocellular carcinoma in Barcelona Clinic Liver Cancer stage C (Double-IA-001): a phase II trial., PMID:40346494
Hepatic artery infusion chemotherapy combined with camrelizumab and apatinib as conversion therapy for patients with unresectable hepatocellular carcinoma: a single-arm exploratory trial., PMID:40335980
Real-World Efficacy and Safety of Anti-PD-1 Antibody Plus Apatinib and Temozolomide for Advanced Acral Melanoma., PMID:40331053
Case Report: Exploring delayed hyperprogressive disease: a case study of post-immunotherapy in lung cancer., PMID:40313962
Efficacy and safety of immune checkpoint inhibitors for brain metastases of non-small cell lung cancer: a systematic review and network meta-analysis., PMID:40308503
Oncolytic adenovirus H101 enhances the anti-tumor effects of PD-1 blockade via CD47 downregulation in tumor cells., PMID:40296909
Efficacy, safety and single-cell analysis of neoadjuvant immunochemotherapy in locally advanced oral squamous cell carcinoma: a phase II trial., PMID:40295492
Combining anti-PD-1 antibodies with surufatinib for gastrointestinal neuroendocrine carcinoma: Two cases report and review of literature., PMID:40290678
Case Report: A long-term response of immunotherapy combined with anti-angiogenesis therapy in a patient with dMMR metastatic colorectal cancer after ICI failure., PMID:40270608
Camrelizumab vs Placebo in Patients With Triple-Negative Breast Cancer-Reply., PMID:40266589
Camrelizumab vs Placebo in Patients With Triple-Negative Breast Cancer., PMID:40266585
Pathologically Complete Response to Camrelizumab and Apatinib in Advanced Cervical Cancer with PTEN, PIK3CA, MTOR, and ARID1A Mutations: A Case Report., PMID:40255685
Post-treatment adverse events ranking in targeted immunotherapy for hepatocellular carcinoma: A network meta-analysis based on risk probability assessment., PMID:40252815
Targeting TIME in advanced hepatocellular carcinoma: Mechanisms of drug resistance and treatment strategies., PMID:40250780
Evaluation of changes in price, volume and expenditure of PD-1 drugs following the government reimbursement negotiation in China: a multiple-treatment period interrupted time series analysis., PMID:40247711
Synchronous triple squamous cell carcinomas of the stomach, skin and gingiva with liver, lung, spleen, kidney, bone and brain metastases: A case report., PMID:40242269
Case Report: Subacute cutaneous lupus erythematosus induced by the anti-PD-1 antibody camrelizumab combined with chemotherapy., PMID:40226630
Evaluation of the efficacy and safety of neoadjuvant immunotherapy in locally advanced esophageal squamous cell carcinoma., PMID:40223985
Plasma proteomic biomarkers predict therapeutic responses in advanced biliary tract cancer patients receiving Camrelizumab plus the GEMOX treatment., PMID:40195413
A case of camrelizumab-induced anaphylaxis and successful rechallenge: a case report and literature review., PMID:40190558
Neoadjuvant camrelizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastroesophageal junction adenocarcinoma: a single-arm, phase 2 trial., PMID:40184004
SMARCA4-deficient non-small cell lung cancer with metastasis to the sigmoid colon: a case report., PMID:40158181
Efficacy and safety of radiotherapy versus transarterial chemoembolization in combination with lenvatinib and camrelizumab for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: a multicenter study., PMID:40140192
Cost-effectiveness of PD-1 inhibitors combined with chemotherapy for first-line treatment of oesophageal squamous cell carcinoma in China: a comprehensive analysis., PMID:40131366
Camrelizumab-based therapies for the treatment of advanced lung cancer: a prospective, open-label, multicenter, observational, real-world study., PMID:40124372
Correlation Between Treatment-Related Adverse Events and Efficacy of Camrelizumab in Combination With Apatinib in Patients With Unresectable Hepatocellular Carcinoma., PMID:40123149
Long-term survival in a patient with ruptured advanced hepatocellular carcinoma treated with nutritional therapy combined with apatinib and camrelizumab: a case report., PMID:40121613
Comparative outcomes of first-line PD-1/PD-L1 inhibitors plus chemotherapy for advanced squamous non-small cell lung cancer: a systematic review and network meta-analysis of randomized clinical trials., PMID:40114962