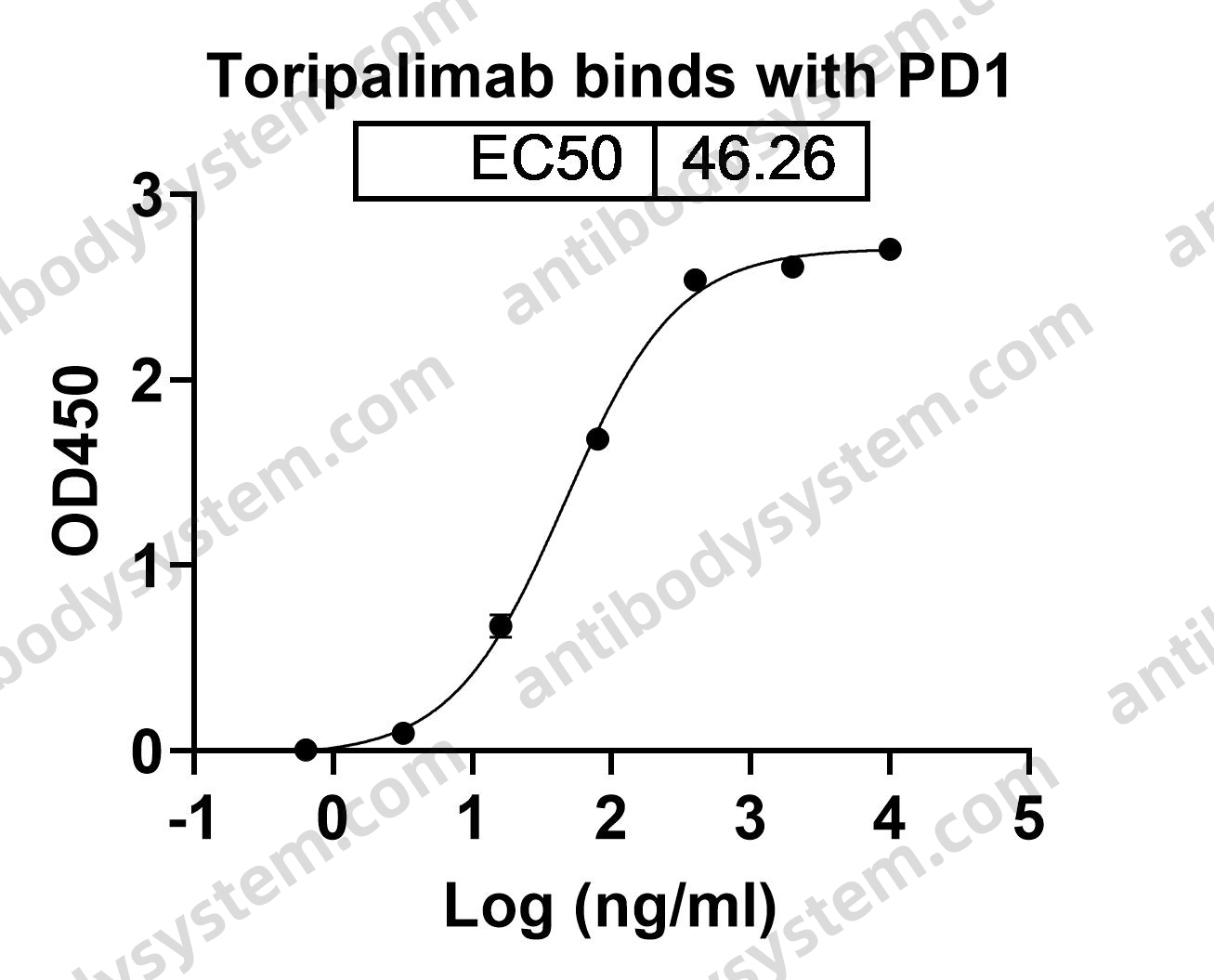
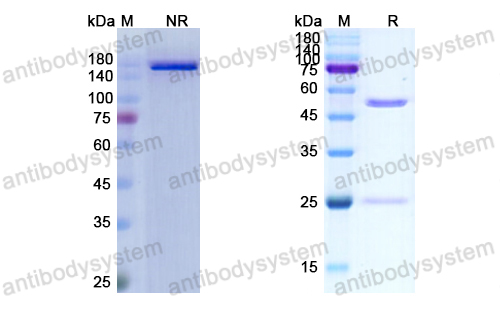
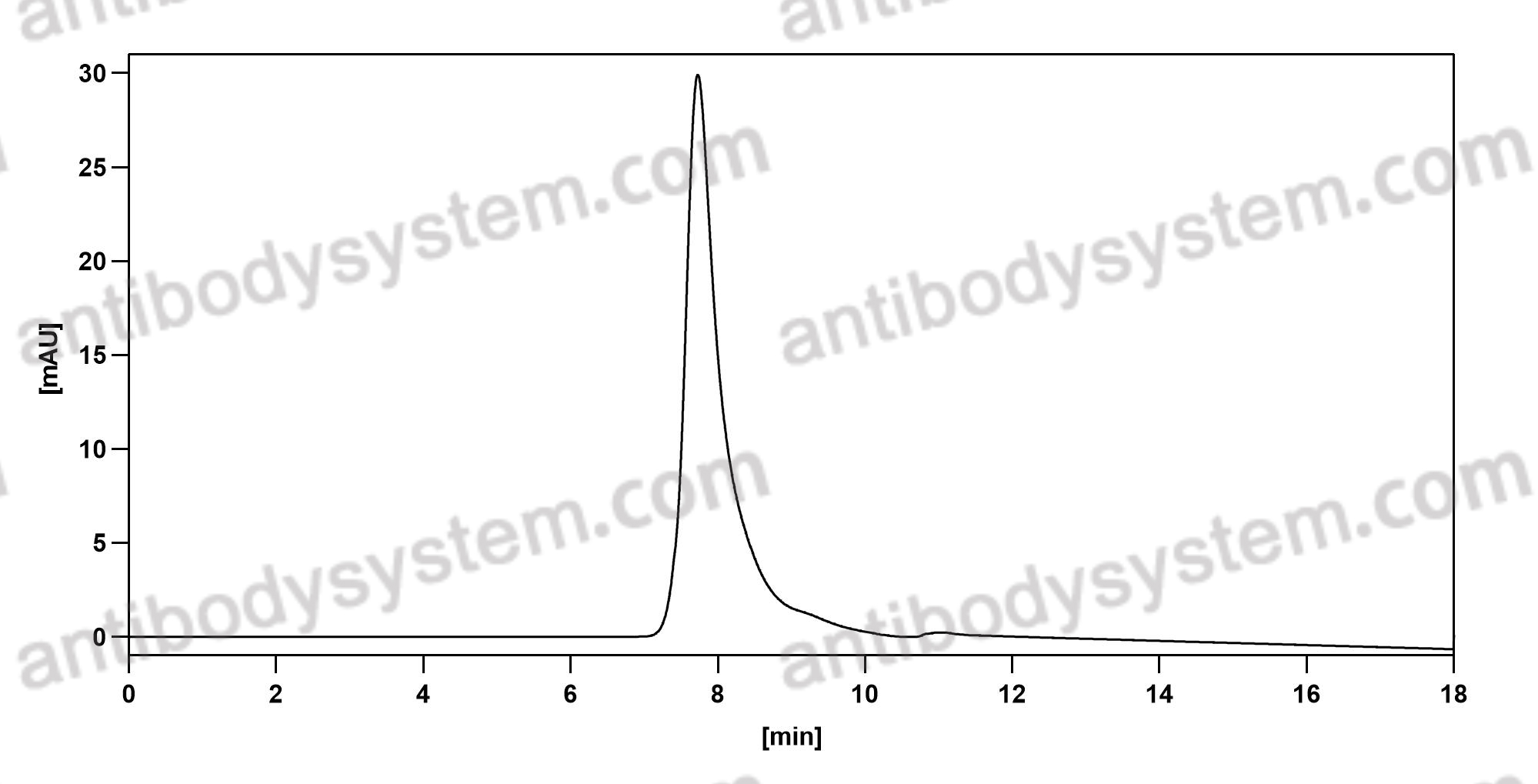
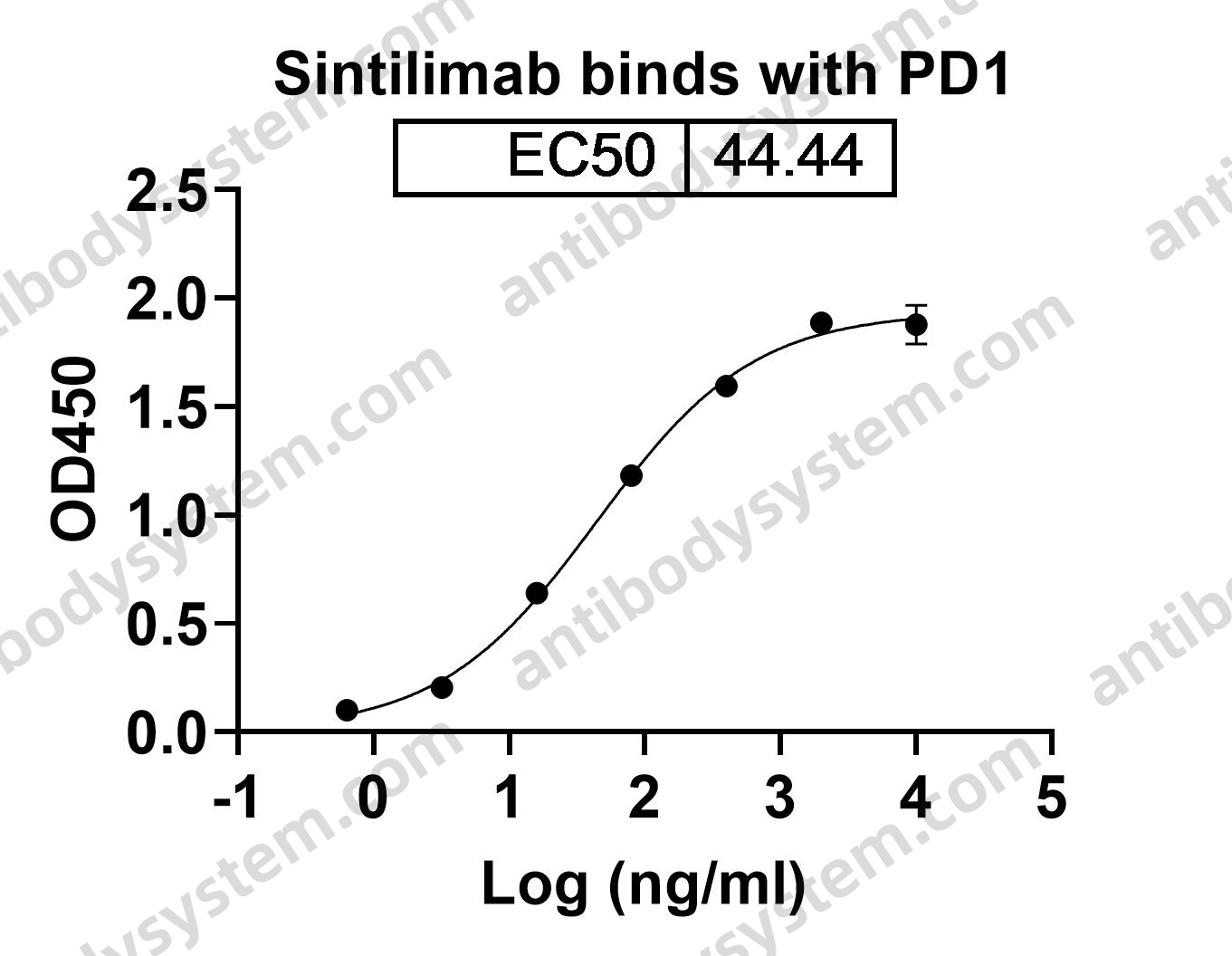
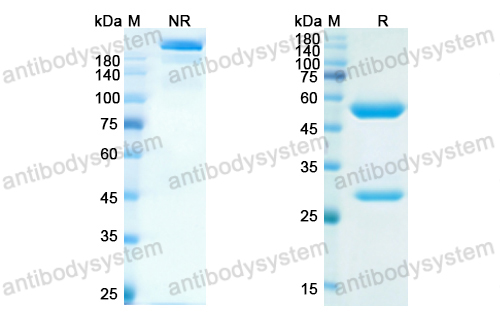
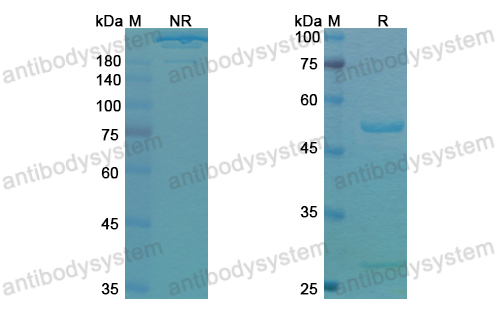
Toripalimab for the treatment of melanoma, PMID: 32406293
Toripalimab: First Global Approval, PMID: 30805896
Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432, PMID: 31236579
Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G 4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial, PMID: 31403867
Effectiveness and safety of toripalimab, camrelizumab, and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients, PMID: 33241036
Efficacy, Safety, and Biomarkers of Toripalimab in Patients with Recurrent or Metastatic Neuroendocrine Neoplasms: A Multiple-Center Phase Ib Trial, PMID: 32086343
Toripalimab-Induced Dermatomyositis in a Patient with Metastatic Melanoma, PMID: 32445174
Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial, PMID: 32321714
Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non-Small Cell Lung Cancer: A Phase 1 Trial, PMID: 33017026
A phase I study of toripalimab, an anti-PD-1 antibody, in patients with refractory malignant solid tumors, PMID: 32589350
Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study, PMID: 32224416
Safety and efficacy of toripalimab in advanced gastric cancer: A new clinical trial bringing hope for immunotherapy in gastric cancer, PMID: 32277740
Successful treatment of advanced pulmonary sarcomatoid carcinoma with the PD-1 inhibitor toripalimab: A case report, PMID: 32943323
Efficacy of chemotherapy combined with toripalimab in PD-L1-positive and high tumor mutation burden pancreatic acinar cell carcinoma: case report, PMID: 33345750
Withdrawn: Effectiveness and safety of toripalimab, camrelizumab and sintilimab in a real-world cohort of hepatitis B virus associated hepatocellular carcinoma patients, PMID: 32627214
Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: a systematic review, PMID: 32489431
Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy, PMID: 30892132
Durable Response and Good Tolerance to the Triple Combination of Toripalimab, Gemcitabine, and Nab-Paclitaxel in a Patient With Metastatic Pancreatic Ductal Adenocarcinoma, PMID: 32636837
Study protocol for an open-label, single-arm, phase Ib/II study of combination of toripalimab, nab-paclitaxel, and gemcitabine as the first-line treatment for patients with unresectable pancreatic ductal adenocarcinoma, PMID: 32646394
A phase II, single-centre trial of neoadjuvant toripalimab plus chemotherapy in locally advanced esophageal squamous cell carcinoma, PMID: 33282388
Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial, PMID: 34341578
Complete response induced by anti-PD-1-based immunotherapy with toripalimab in a patient with locally advanced lung adenocarcinoma who failed rapidly after concurrent chemoradiotherapy: A case report, PMID: 32893899
Complete and enduring response in an elderly patient with repeated recurrent gingival squamous cell carcinoma treated by combined toripalimab and single agent chemotherapy: a case report, PMID: 33183019
Correction: Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial, PMID: 32934029
Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients, PMID: 30642373
Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma, PMID: 33854567
An advanced non-small cell lung cancer patient with epidermal growth factor receptor sensitizing mutation responded to toripalimab in combination with chemotherapy after resistance to osimertinib: a case report, PMID: 32420075
Pretransplant use of toripalimab for hepatocellular carcinoma resulting in fatal acute hepatic necrosis in the immediate postoperative period, PMID: 33744409
Remarkable Response of Toripalimab Combined with Chemotherapy in Sarcomatoid Carcinoma of Palatine Tonsil: A Case Report, PMID: 33727822
Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02), PMID: 33492986
PD-1 Blockade in Chinese versus Western Patients with Melanoma, PMID: 32487680
Efficacy and safety of toripalimab combined with doxorubicin as first-line treatment for metastatic soft tissue sarcomas: an observational study, PMID: 34001702
Discovery of New Immune Checkpoints: Family Grows Up, PMID: 32185707
Coexistence of oral mucous membrane pemphigoid and lichenoid drug reaction: a case of toripalimab-triggered and pembrolizumab-aggravated oral adverse events, PMID: 34238713
A phase III study on neoadjuvant chemotherapy versus neoadjuvant toripalimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: Henan Cancer Hospital Thoracic Oncology Group 1909 (HCHTOG1909), PMID: 33553366
Positron Emission Tomography Imaging of Programmed Death 1 Expression in Cancer Patients Using 124I-Labeled Toripalimab: A Pilot Clinical Translation Study, PMID: 33512952
Landscape of immune checkpoint inhibitor-related adverse events in Chinese population, PMID: 32968172
Evaluation of 124 I-JS001 for hPD1 immuno-PET imaging using sarcoma cell homografts in humanized mice, PMID: 32874831
Primary gastric melanoma in a young woman: A case report, PMID: 33392326
p.P476S mutation of RBPJL inhibits the efficacy of anti-PD-1 therapy in oesophageal squamous cell carcinoma by blunting T-cell responses, PMID: 32994998
Rapid advances in research on and development of anticancer drugs in China, PMID: 31511442
Prior anti-PD-1 therapy as a risk factor for life-threatening peri-engraftment respiratory distress syndrome in patients undergoing autologous stem cell transplantation, PMID: 33273659
Favorable response to immunotherapy in a pancreatic neuroendocrine tumor with temozolomide-induced high tumor mutational burden, PMID: 33230973
Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma, PMID: 33732355
Genomic Landscape of Chinese Clear Cell Renal Cell Carcinoma Patients With Venous Tumor Thrombus Identifies Chromosome 9 and 14 Deletions and Related Immunosuppressive Microenvironment, PMID: 34249685
Development and Validation of a Contrast-Enhanced CT-Based Radiomics Nomogram for Prediction of Therapeutic Efficacy of Anti-PD-1 Antibodies in Advanced HCC Patients, PMID: 33488622
Combination of Anti-Programmed Death 1 Therapy and Apatinib for a Patient with Hepatocellular Carcinoma and Brain Metastasis: Case Report and Literature Review, PMID: 32534262
Effective Treatment with PD-1 Antibody, Chidamide, Etoposide, and Thalidomide (PCET) for Relapsed/Refractory Natural Killer/T-Cell Lymphoma: A Report of Three Cases, PMID: 32801749
Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis, PMID: 34067837
Antiangiogenesis Combined with Immunotherapy to Treat Advanced Small-Cell Carcinoma of the Esophagus Resistant to Chemotherapy: According to the Guidance of Next-Generation Sequencing, PMID: 33688208
Four-year outcomes with perioperative toripalimab plus chemotherapy in resectable stage III non-small cell lung cancer (NeoTAP01 study)., PMID:40529778
Perioperative toripalimab and chemotherapy in resectable non-small cell lung cancer: another weapon in the armamentarium., PMID:40529735
Efficacy and safety of HBM4003 combined with toripalimab in refractory neuroendocrine neoplasms: a multicenter, phase II study., PMID:40521168
Efficacy and toxicity of PD-1 inhibitor combined with induction chemotherapy for locally advanced laryngeal and hypopharyngeal cancers., PMID:40520879
GFH018 and toripalimab combination therapy for previously treated recurrent or metastatic nasopharyngeal carcinoma: Results from a phase 1b/2 study., PMID:40512241
Efficacy and safety of SABR/partial-SABR combined with axitinib and toripalimab in recurrent or metastatic renal cell carcinoma: Preliminary results from a prospective phase 2 trial., PMID:40503038
First-line toripalimab plus chemotherapy versus chemotherapy for advanced esophageal squamous cell carcinoma: A cost-effectiveness analysis., PMID:40493606
Case Report: Remarkable and sustained remission of an anaplastic thyroid carcinoma patient to the combined treatment of multimodal radiotherapy, anlotinib and toripalimab., PMID:40469189
First-line treatment with a combination of immunotherapy, anti-EGFR monoclonal antibodies, and chemotherapeutics for unresectable left KRAS/BRAF wild-type microsatellite-stable colorectal cancer: a case report., PMID:40460045
Neoadjuvant toripalimab combined with chemotherapy in locally advanced HNSCC., PMID:40458723
Cost-effectiveness analysis of Toripalimab regimen for extensive-stage small-cell lung cancer in China and America., PMID:40453073
Anlotinib plus toripalimab as a first-line treatment in patients with advanced gastric cancer and performance status 2: the phase II APICAL-GC trial., PMID:40450051
Primary adenocarcinoma of the renal pelvis: A case report., PMID:40441222
Phase 1b/2 study of ADG106, a 4-1BB/CD137 agonist, in combination with toripalimab in patients with advanced solid tumors., PMID:40421181
Efficacy of toripalimab in combination with anlotinib in recurrent undifferentiated pleomorphic sarcoma of the sinonasal region: a case report with biomarker analysis., PMID:40416963
Toripalimab plus bevacizumab versus sorafenib as first-line treatment for advanced hepatocellular carcinoma (HEPATORCH): a randomised, open-label, phase 3 trial., PMID:40409323
Tifcemalimab as monotherapy or in combination with toripalimab in patients with relapsed/refractory lymphoma: a Phase I trial., PMID:40379637
Phase I/II Study of Tifcemalimab, an Anti-B and T-lymphocyte Attenuator Antibody, in Combination with Toripalimab in Previously Treated Advanced Lung Cancer., PMID:40378060
Porustobart (HBM4003) plus toripalimab as second-line therapy in patients with advanced hepatocellular carcinoma: a multicenter, open-label, phase I study., PMID:40378055
Case Report: Combined PD-1 and tyrosine kinase blockade stabilizes refractory pancreatic cancer guided by the spatial structure of tumor immune microenvironment., PMID:40376004
Toripalimab-Associated Arthritis and Peripheral Neuropathy., PMID:40366363
Evaluating the efficacy and safety of bladder-sparing regimen with Disitamab Vedotin combined with Toripalimab and pelvic lymph node dissection in muscle-invasive bladder cancer patients: study protocol of a multicenter single-arm phase II trial., PMID:40361038
Clinical manifestations and risk factors of immune-related thyroid adverse events in patients treated with PD-1 inhibitors: a case-control study., PMID:40356893
Aggressive recurrent intestinal-type adenocarcinoma of the nasal cavity: a case report and literature review., PMID:40303364
Diagnosis and treatment of pulmonary lymphoepithelioma-like carcinoma: A case report., PMID:40290685
Perioperative the BTLA inhibitor (tifcemalimab) combined with toripalimab and chemotherapy for resectable locally advanced thoracic esophageal squamous cell carcinoma trial (BT-NICE trial): a prospective, single-arm, exploratory study., PMID:40276504
Both complete response and long-term survival after combination therapy with toripalimab in a patient with meta-oligometastases cervical cancer: a case report., PMID:40260238
Safety and efficacy of neoadjuvant toripalimab plus chemotherapy followed by chemoradiotherapy for locally advanced esophageal squamous cell carcinoma in China (GASTO 1071): a non-randomised, two-cohort, phase 2 trial., PMID:40242562
Evaluation of the efficacy and safety of toripalimab combination therapy for treatment of advanced gastric cancer: a meta-analysis., PMID:40226111
Neoadjuvant toripalimab plus nimotuzumab combined with taxol-based chemotherapy in locally advanced penile squamous cell carcinoma., PMID:40215977
Conversion therapy combined with ALPPS for the treatment of intrahepatic cholangiocarcinoma: a case report., PMID:40190549
[Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma]., PMID:40189973
Cost-effectiveness analysis of toripalimab combined with nab-paclitaxel as a first-line treatment for advanced TNBC in the US., PMID:40168440
[Toripalimab in combination with cisplatin and gemcitabine in recurrent or metastatic nasopharyngeal carcinomas (UCNT RM) in first-line treatment]., PMID:40140319
Efficacy and safety of autologous CIK cell therapy plus Toripalimab with or without chemotherapy in advanced NSCLC: A phase II study., PMID:40135473
Cost-effectiveness of PD-1 inhibitors combined with chemotherapy for first-line treatment of oesophageal squamous cell carcinoma in China: a comprehensive analysis., PMID:40131366
Immune-mediated hepatitis caused by toripalimab: A case report., PMID:40116194
Clinical Manifestations and Risk Factors of Liver Injury Induced by PD-1 Inhibitors in Patients with Malignancies: A Case-Control Study., PMID:40092894
Effect of Early Tumor Shrinkage and Depth of Response on the Clinical Outcomes of Patients with Unresectable Hepatocellular Carcinoma Treated with Transcatheter Arterial Chemoembolization and Lenvatinib plus PD-1 Inhibitors., PMID:40073848
A patient with penile metastasis secondary to small cell lung cancer successfully treated with PD-1 inhibitors and chemotherapy: a case report and literature review., PMID:40071100
Efficacy and safety of targeted therapy and immunotherapy in advanced vulvar squamous cell carcinoma: A scoping review., PMID:40068805
Are all programmed cell death protein 1 inhibitors the same?, PMID:40027132
Long-Term and Sustained Remission of Advanced Triple-Negative Breast Cancer with Large Chest Wall Lesions after Transient Chemoimmunotherapy: A Case Report., PMID:39980521
A phase I dose-escalation and expansion study of RMX1002, a selective E-type prostanoid receptor 4 antagonist, as monotherapy and in combination with anti-PD-1 antibody in advanced solid tumors., PMID:39976872
Toripalimab: A new age in fighting Nasopharyngeal Carcinoma., PMID:39948809
Extended survival of a patient with gastrointestinal multiple malignancies managed with anti-PD-1 immunotherapy: a case report., PMID:39935272
Efficacy and Safety of Low-Dose Lenvatinib and Toripalimab in Patients With Recurrent Platinum-Resistant Ovarian Cancer: Study Protocol of a Multicenter, Open-Label, Single-Arm, Phase II Clinical Trial., PMID:39931671
Long term survival following cryoablation with adjuvant Toripalimab for anorectal malignant melanoma: a case report., PMID:39926280
Anti-LAG-3 antibody LBL-007 plus anti-PD-1 antibody toripalimab in advanced nasopharyngeal carcinoma and other solid tumors: an open-label, multicenter, phase Ib/II trial., PMID:39920751
Efficacy of radiolabelled PD-L1-targeted nanobody in predicting and evaluating the combined immunotherapy and chemotherapy for resectable non-small cell lung cancer., PMID:39912938