Catalog No.
DHH02203
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
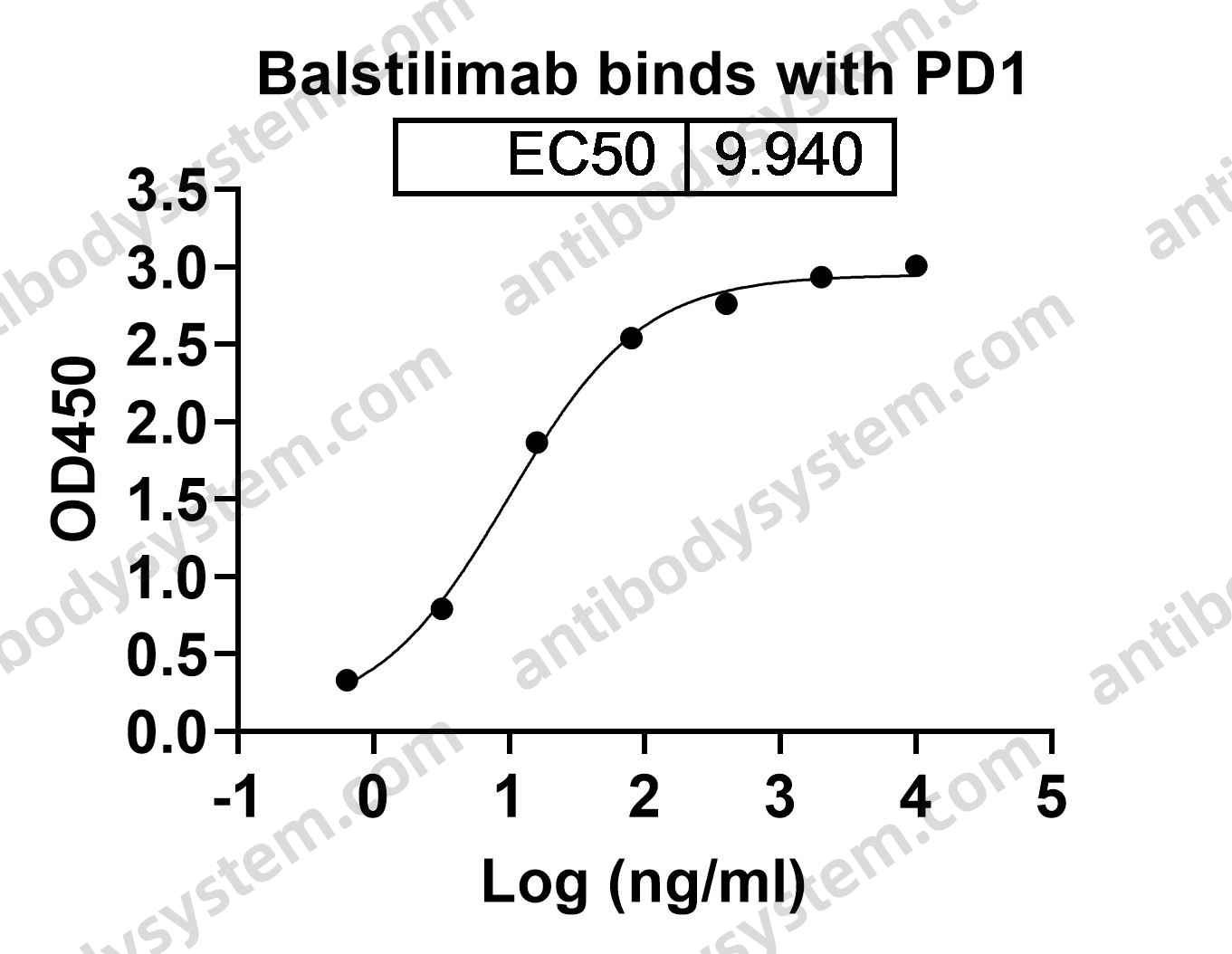
Programmed cell death protein 1, Protein PD-1, hPD-1, PD1, PDCD1, CD279
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
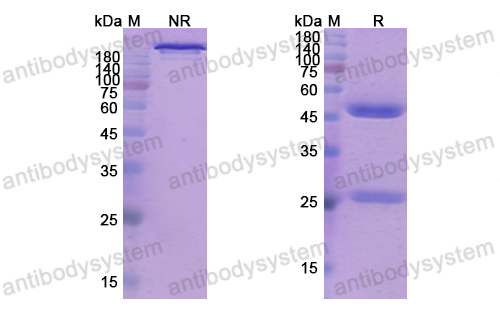
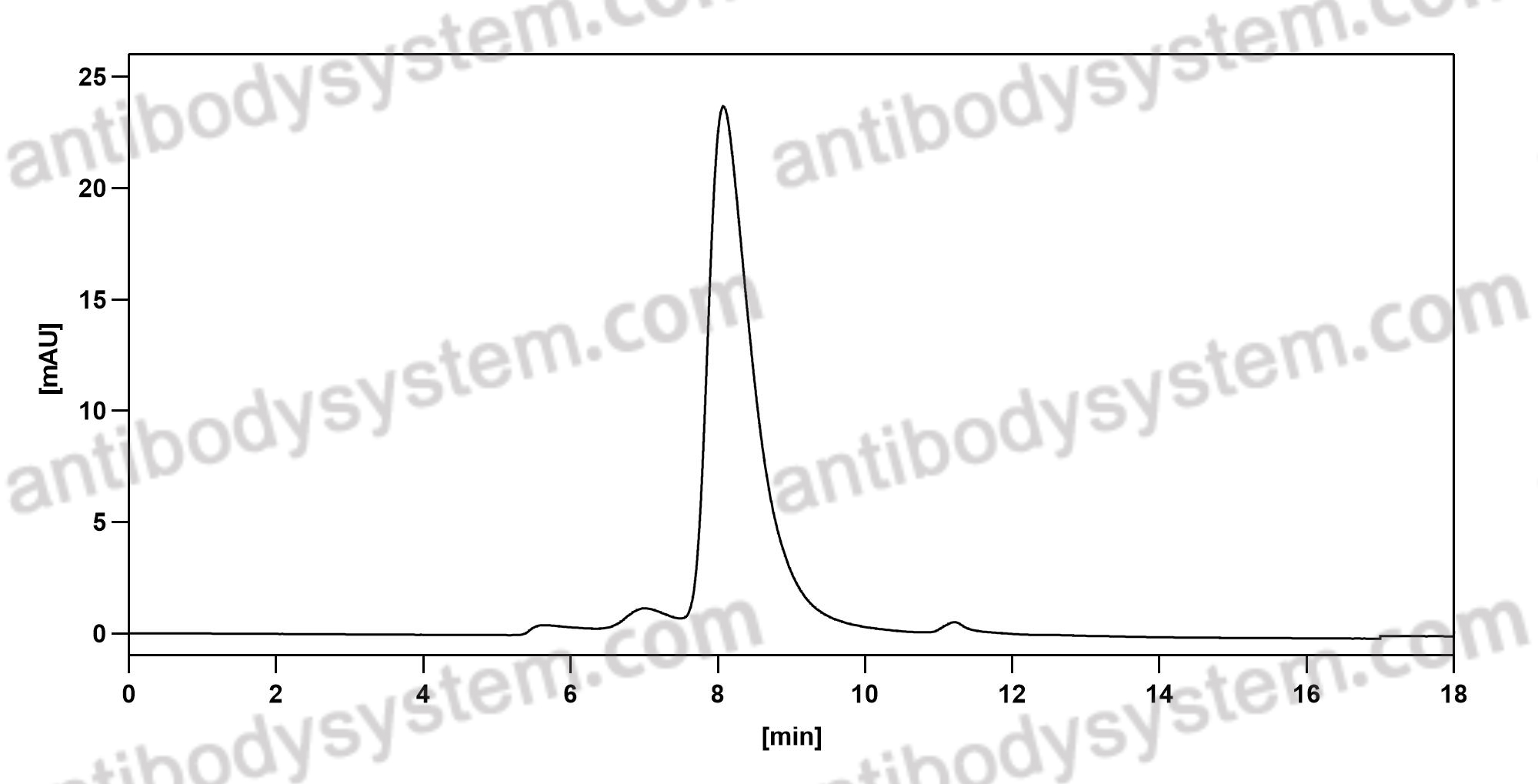
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q15116
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
AGEN2034, CAS: 2230167-06-1
Clone ID
Balstilimab
Antibodies to watch in 2020, PMID: 31847708
Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor, PMID: 33500548
Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer, PMID: 34452745
RaPiDS (GOG-3028): randomized Phase II study of balstilimab alone or in combination with zalifrelimab in cervical cancer, PMID: 34409858
A single arm phase 2 trial of doxorubicin plus zalifrelimab (anti-CTLA-4 antibody) and balstilimab (anti-PD-1 antibody) in advanced/metastatic soft tissue sarcomas., PMID:40378054
Botensilimab (Fc-enhanced anti-cytotoxic lymphocyte-association protein-4 antibody) Plus Balstilimab (anti-PD-1 antibody) in Patients With Relapsed/Refractory Metastatic Sarcomas., PMID:39869830
The Prostaglandin EP4 Antagonist Vorbipiprant Combined with PD-1 Blockade for Refractory Microsatellite-Stable Metastatic Colorectal Cancer: A Phase Ib/IIa Trial., PMID:39620921
Vitamin A Metabolism and Resistance of Hepatic Metastases to Immunotherapy., PMID:39363636
Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial., PMID:38871975
Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer., PMID:37731056
Beyond Platinum, ICIs in Metastatic Cervical Cancer: A Systematic Review., PMID:36497437
Abscobal Effect of Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer., PMID:35486885
Current and emerging immunotherapies for recurrent cervical cancer., PMID:35120091
Balstilimab and other immunotherapy for recurrent and metastatic cervical cancer., PMID:35092506
Dual PD-1 and CTLA-4 Checkpoint Blockade Using Balstilimab and Zalifrelimab Combination as Second-Line Treatment for Advanced Cervical Cancer: An Open-Label Phase II Study., PMID:34932394
Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer., PMID:34452745
RaPiDS (GOG-3028): randomized Phase II study of balstilimab alone or in combination with zalifrelimab in cervical cancer., PMID:34409858
Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor., PMID:33500548
Antibodies to watch in 2020., PMID:31847708