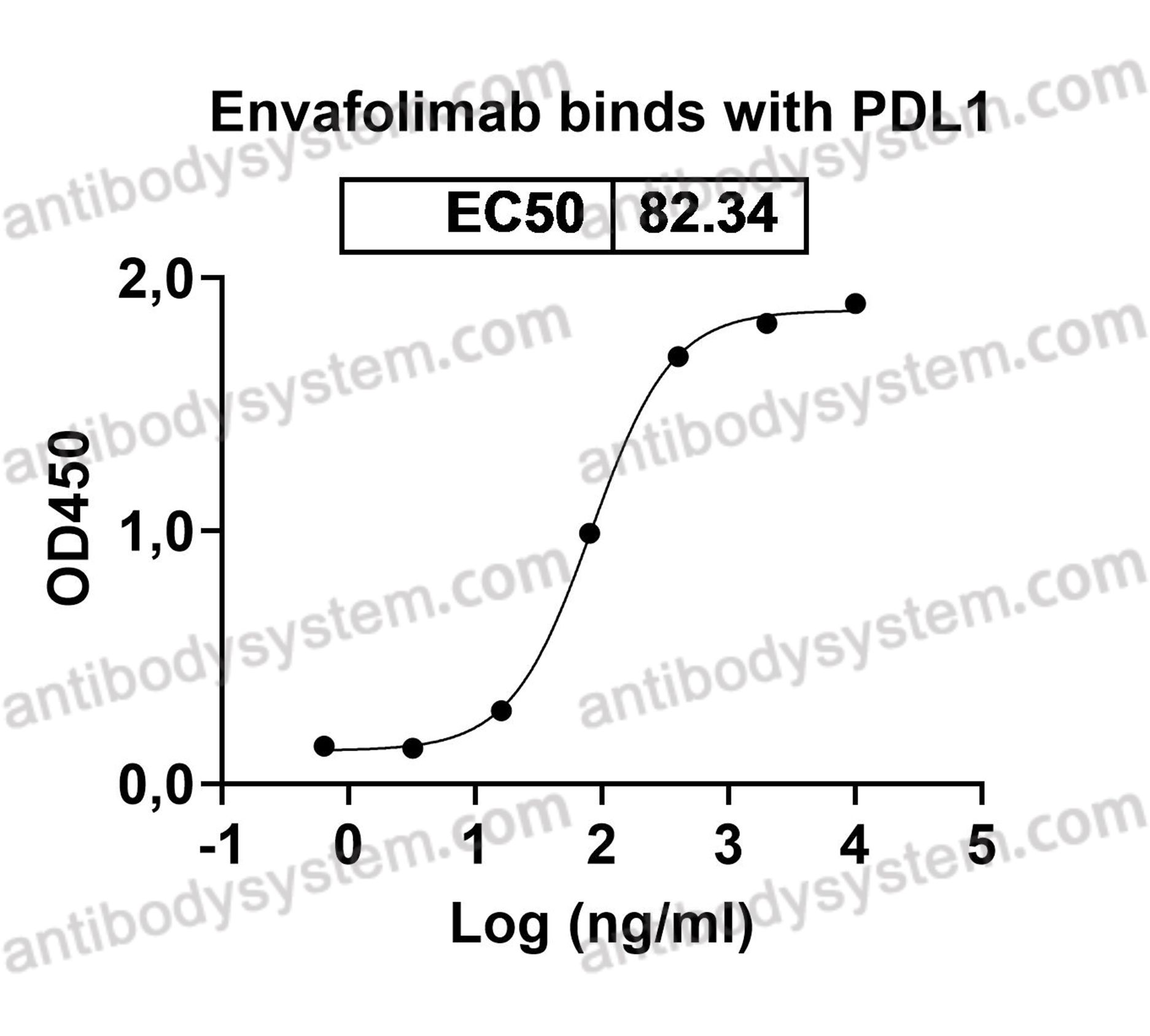
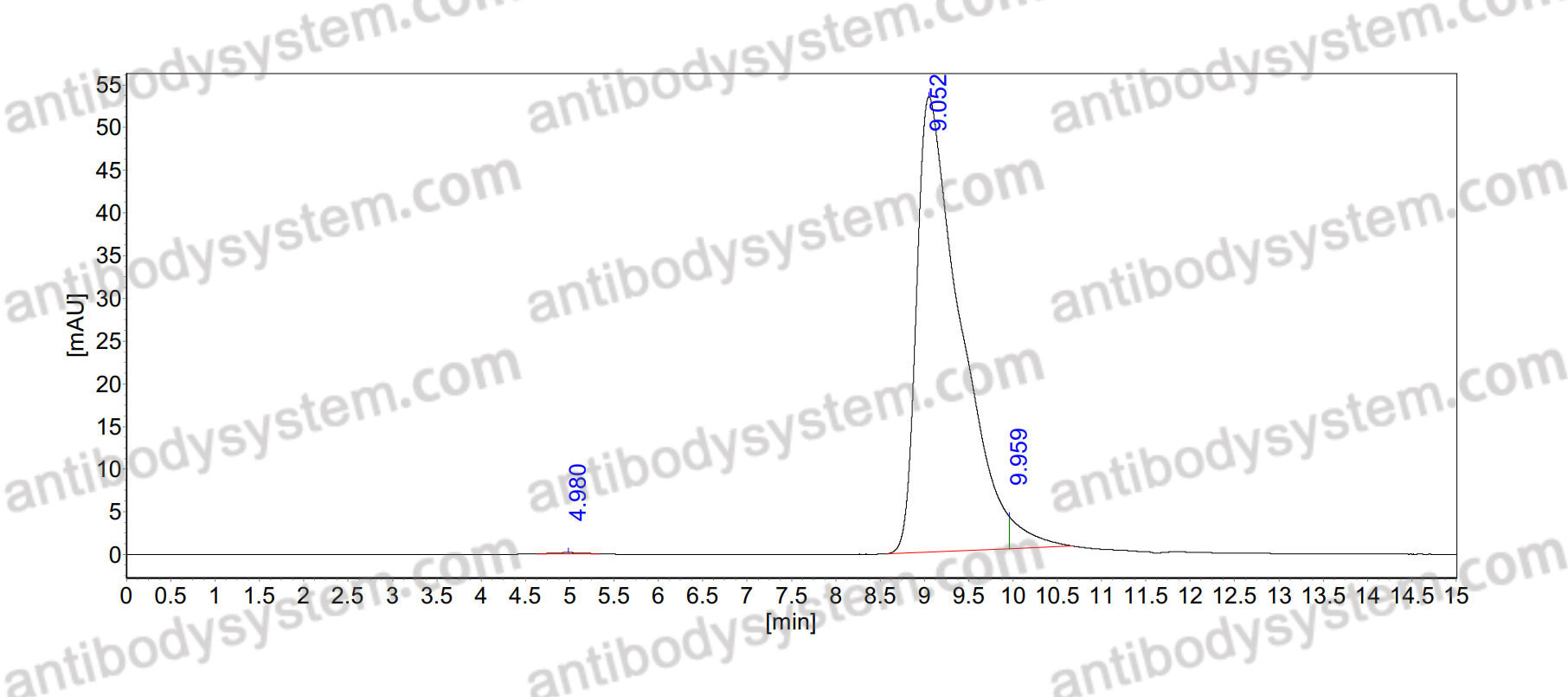
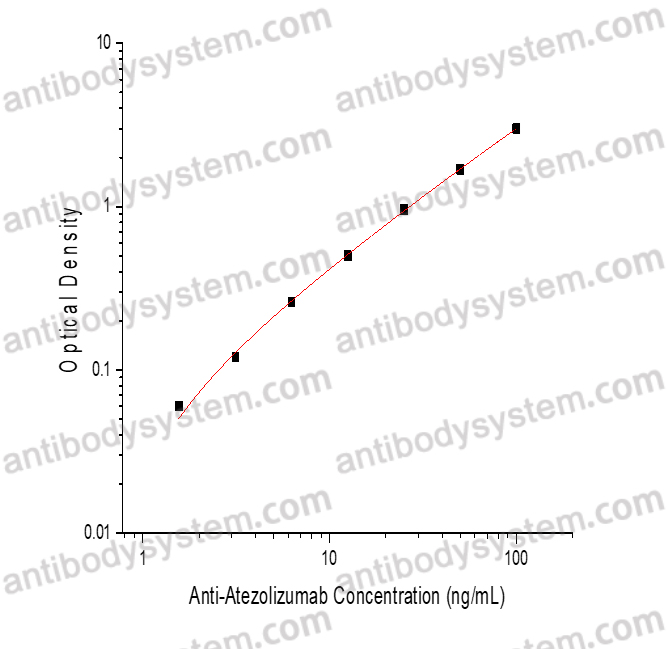
Catalog No.
DHJ70104
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Chimeric
Isotype
(VH-CH2-CH3)2
Clonality
Monoclonal
Target
B7-H1, Programmed cell death 1 ligand 1, PDCD1 ligand 1, PDCD1L1, B7 homolog 1, PDCD1LG1, PDL1, hPD-L1, Programmed death ligand 1, B7H1, PD-L1, CD274
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9NZQ7
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
KN-035, CAS: 2102192-68-5
Clone ID
Envafolimab
Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors, PMID: 34154614
First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors, PMID: 33973293
Safety and Efficacy of the First Subcutaneous ICI, Envafolimab, in the Treatment of Advanced Lung Cancer Patients: A Real-World Study., PMID:40524516
A study on the effectiveness and safety of interventional embolization using drug-loaded microspheres in combination with sorafenib and envafolimab for intermediate and advanced renal cell carcinoma., PMID:40510141
Immunotherapy for primary squamous cell carcinoma of the liver: A case report., PMID:40242270
Case report: A case of type 1 diabetes with diabetic ketoacidosis induced by envafolimab treatment in hepatocellular carcinoma., PMID:39958361
Anti-PD-L1 envafolimab combined with anti-VEGF suvemcitug in pretreated solid tumors and hepatocellular carcinoma: an open-label phase II study with safety run-in stage., PMID:39883265
Efficacy and safety of the combination of anlotinib and envafolimab in the treatment of unresectable or metastatic liposarcoma: findings from a single-center retrospective study., PMID:39868378
The efficacy and safety of suvemcitug, envafolimab, and FOLFIRI in microsatellite-stable or mismatch repair-proficient colorectal cancer: preliminary results of a phase 2 study., PMID:39836256
Efficacy and safety of the combination of envafolimab and lenvatinib in unresectable hepatocellular carcinoma: a single-arm, multicentre, exploratory phase II clinical study., PMID:39690337
A Case Report of Envafolimab in the Treatment of Microsatellite Stable (MSS) Metastatic Colon Cancer., PMID:39620139
Case report: Combination therapy of envafolimab with endostar for advanced non-small cell lung cancer with low PD-L1 expression., PMID:39575420
Envafolimab plus lenvatinib and transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a prospective, single-arm, phase II study., PMID:39384742
β-glucan combined with Envafolimab and Endostar as immune rechallenge for metastatic non-small cell lung cancer., PMID:39271997
Neoadjuvant envafolimab in a patient with MSI-H/dMMR colon cancer: a case report and literature review., PMID:39259508
Single domain antibody: Development and application in biotechnology and biopharma., PMID:39166870
Efficacy and safety of combining short-course neoadjuvant chemoradiotherapy with envafolimab in locally advanced rectal cancer patients with microsatellite stability: a phase II PRECAM experimental study., PMID:39093871
Envafolimab Inhibits the Growth of Gastric Cancer Cells with Low PD-L1 Expression through the DDX20/NF-κB/TNF-α Signaling Pathway., PMID:39021191
Model-informed drug development of envafolimab, a subcutaneously injectable PD-L1 antibody, in patients with advanced solid tumors., PMID:38982653
A randomized phase 2b study of subcutaneous PD-L1 antibody ASC22 in virally suppressed patients with chronic hepatitis B who are HBeAg-negative., PMID:38976867
Correction to: Efficacy and Safety of Neoadjuvant Subcutaneous Envafolimab in dMMR/MSI-H Locally Advanced Colon Cancer., PMID:38833122
Efficacy and Safety of Neoadjuvant Subcutaneous Envafolimab in dMMR/MSI-H Locally Advanced Colon Cancer., PMID:38691294
Case report: Envafolimab combined with Endostar in the treatment of advanced non-small cell lung cancer with malignant pleural effusion., PMID:38638859
Chidamide plus envafolimab as subsequent treatment in advanced non-small cell lung cancer patients resistant to anti-PD-1 therapy: A multicohort, open-label, phase II trial with biomarker analysis., PMID:38597130
Case report: Envafolimab causes local skin necrosis., PMID:38585260
Plasma sPD-L1 and VEGF levels are associated with the prognosis of NSCLC patients treated with combination immunotherapy., PMID:38386011
A retrospective study on the efficacy and safety of Envafolimab, a PD-L1 inhibitor, in the treatment of advanced malignant solid tumors., PMID:38357311
Imaging diagnosis and efficacy monitoring by [89Zr]Zr-DFO-KN035 immunoPET in patients with PD-L1-positive solid malignancies., PMID:38164149
[Occurrence of small cell lung cancer after long-term benefit from envafolimab for advanced MSI-H/dMMR colon cancer]., PMID:37968086
[A phase I study of subcutaneous envafolimab (KN035) monotherapy in Chinese patients with advanced solid tumors]., PMID:37875426
Efficacy and safety of envafolimab in the treatment of advanced dMMR/MSI‑H solid tumors: A single‑arm meta‑analysis., PMID:37545619
Case report: A case of complete clinical response in a patient experiencing high microsatellite instability unresectable colon cancer being treated with a PD-L1 inhibitor after interstitial pneumonia., PMID:36998453
Envafolimab combined with chemotherapy in the treatment of combined small cell lung cancer: A case report., PMID:36874434
Noninvasive Imaging of Tumor PD-L1 Expression Using [99mTc]Tc-Labeled KN035 with SPECT/CT., PMID:36541699
Envafolimab - first PD-1/PD-L1 antibody to be administered by subcutaneous injection for microsatellite instability-high or deficient mismatch repair advanced solid tumors., PMID:36124972
Phase I study of envafolimab (KN035), a novel subcutaneous single-domain anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors., PMID:35932387
Design, synthesis, and preclinical evaluation of a novel bifunctional macrocyclic chelator for theranostics of cancers., PMID:35347438
Envafolimab: First Approval., PMID:35122636
Residue-specific binding mechanisms of PD-L1 to its monoclonal antibodies by computational alanine scanning., PMID:34259259
Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors., PMID:34154614
First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors., PMID:33973293