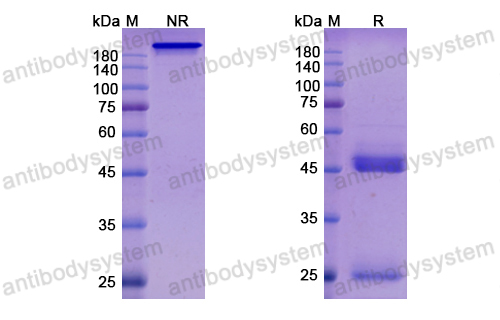
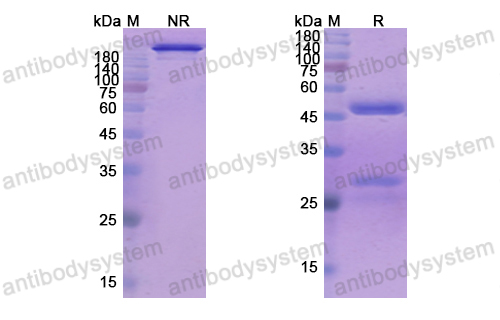
Catalog No.
DHA29102
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-nd
Clonality
Monoclonal
Target
TRAILR2, TRAIL-R2, Death receptor 5, CD262, DR5, Tumor necrosis factor receptor superfamily member 10B, ZTNFR9, TRAIL receptor 2, TRICK2, TNFRSF10B, KILLER, TNF-related apoptosis-inducing ligand receptor 2
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
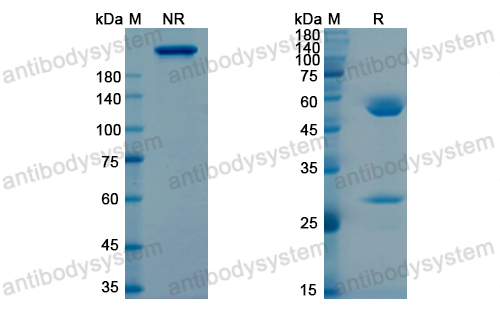
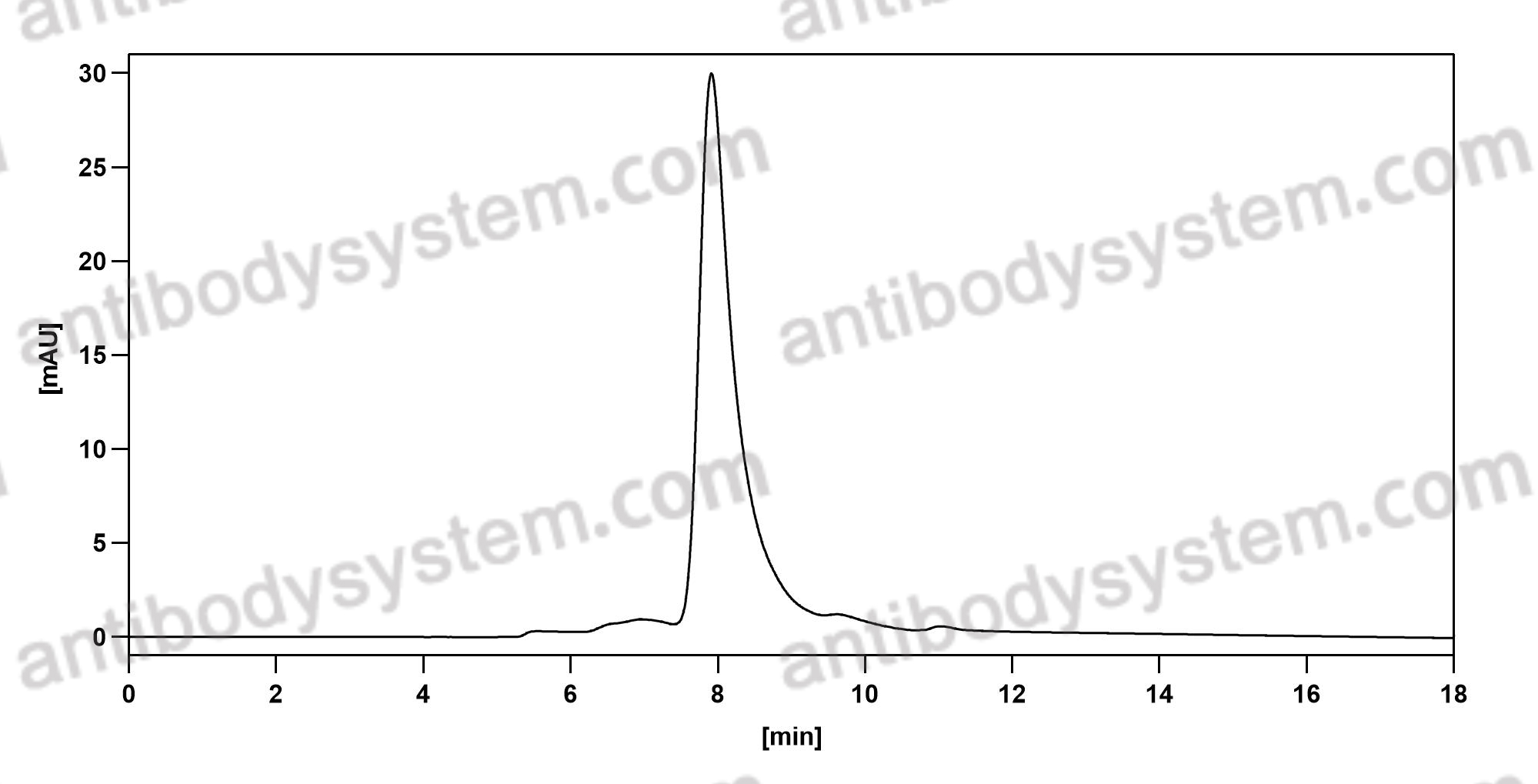
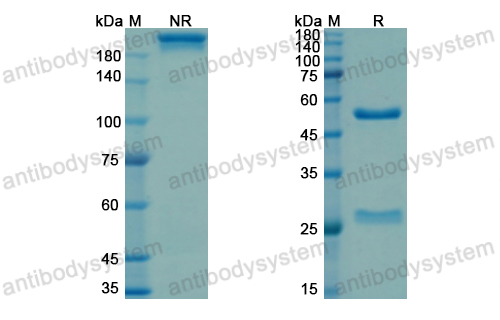
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
O14763
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
CS-1008, TRA-8, CAS: 918127-53-4
Clone ID
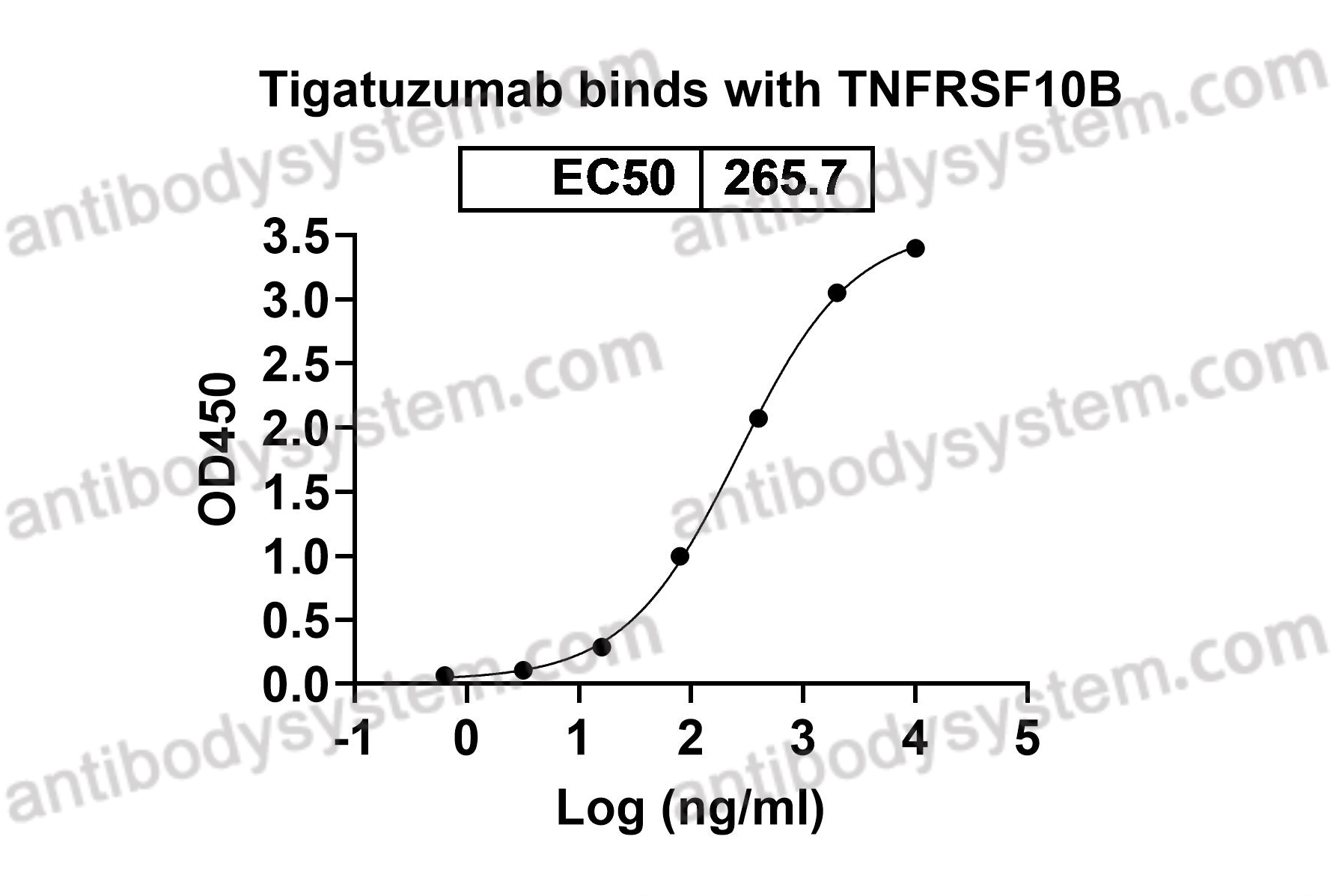
Tigatuzumab
Oral recombinant methioninase increases TRAIL receptor-2 expression to regress pancreatic cancer in combination with agonist tigatuzumab in an orthotopic mouse model, PMID: 32739322
Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study, PMID: 26071796
Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1-dependent inhibition of STAT3, PMID: 22230479
A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naïve metastatic/unresectable non-small cell lung cancer, PMID: 24148258
Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer, PMID: 24403266
Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5), PMID: 20187792
TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer, PMID: 25779953
Death receptors as targets in cancer, PMID: 23638798
Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives, PMID: 27665540
Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer, PMID: 26124477
Molecular imaging of death receptor 5 occupancy and saturation kinetics in vivo by humanized monoclonal antibody CS-1008, PMID: 24045184
Significance of Circulating Tumor Cells in Metastatic Triple-Negative Breast Cancer Patients within a Randomized, Phase II Trial: TBCRC 019, PMID: 25779948
A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model, PMID: 20660600
Sorafenib and its derivative SC-49 sensitize hepatocellular carcinoma cells to CS-1008, a humanized anti-TNFRSF10B (DR5) antibody, PMID: 22978563
Phase I-IV Drug Trials on Hepatocellular Carcinoma in Asian Populations: A Systematic Review of Ten Years of Studies., PMID:39273237
Neutrophils as potential therapeutic targets for breast cancer., PMID:37972723
A patch of positively charged residues regulates the efficacy of clinical DR5 antibodies in solid tumors., PMID:34731630
Oral recombinant methioninase increases TRAIL receptor-2 expression to regress pancreatic cancer in combination with agonist tigatuzumab in an orthotopic mouse model., PMID:32739322
Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives., PMID:27665540
Phase I Imaging and Pharmacodynamic Trial of CS-1008 in Patients With Metastatic Colorectal Cancer., PMID:26124477
Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study., PMID:26071796
TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer., PMID:25779953
Significance of Circulating Tumor Cells in Metastatic Triple-Negative Breast Cancer Patients within a Randomized, Phase II Trial: TBCRC 019., PMID:25779948
Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer., PMID:24403266
A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naïve metastatic/unresectable non-small cell lung cancer., PMID:24148258
Molecular imaging of death receptor 5 occupancy and saturation kinetics in vivo by humanized monoclonal antibody CS-1008., PMID:24045184
Death receptors as targets in cancer., PMID:23638798
Sorafenib and its derivative SC-49 sensitize hepatocellular carcinoma cells to CS-1008, a humanized anti-TNFRSF10B (DR5) antibody., PMID:22978563
Dovitinib sensitizes hepatocellular carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5 antibody, through SHP-1-dependent inhibition of STAT3., PMID:22230479
A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model., PMID:20660600
Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5)., PMID:20187792