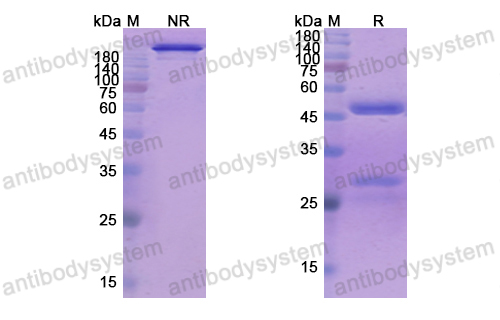
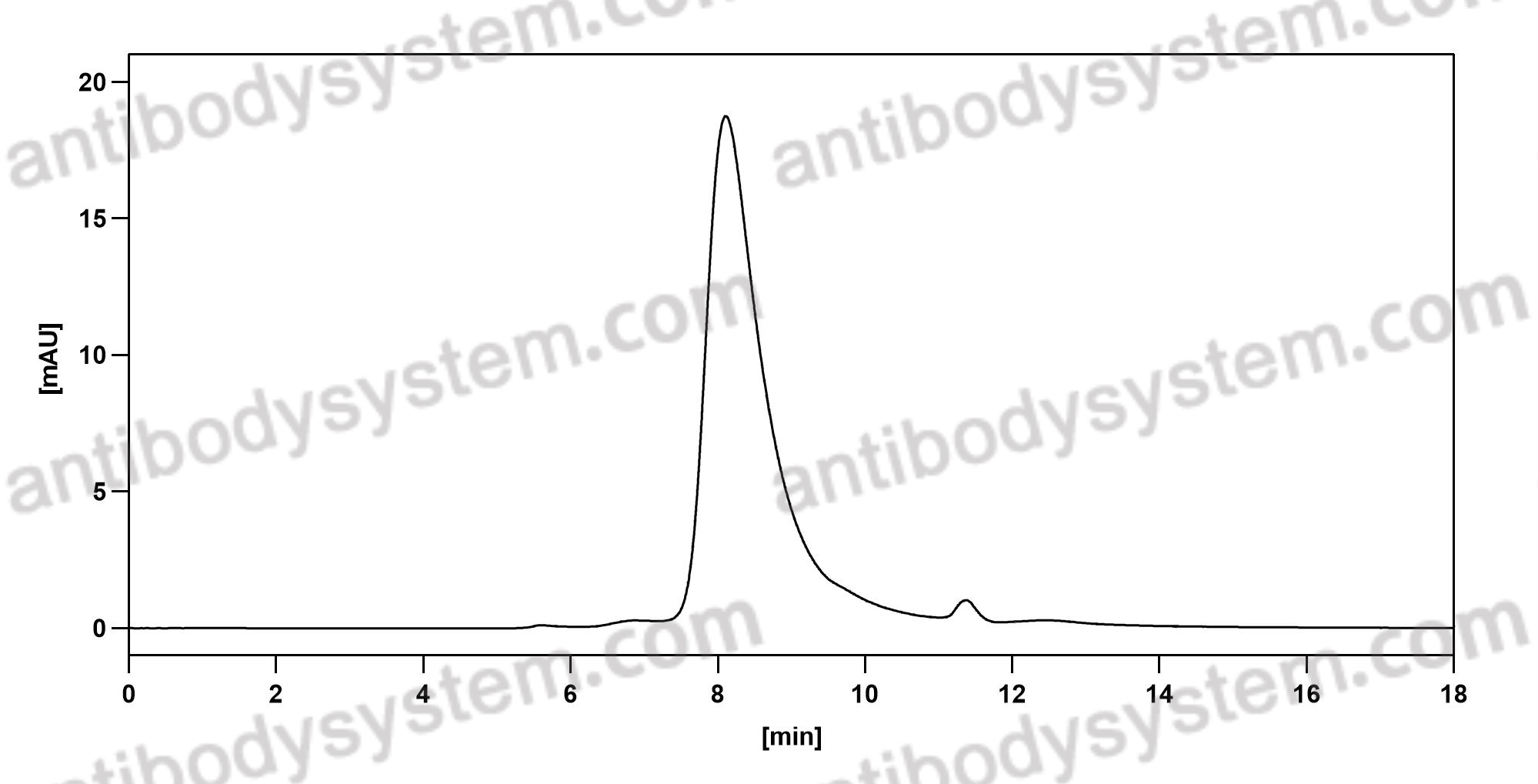
111 In-Labeled mapatumumab, PMID: 20641403
99m Tc-Labeled mapatumumab, PMID: 20641397
Targeting apoptosis: preclinical and early clinical experience with mapatumumab, an agonist monoclonal antibody targeting TRAIL-R1, PMID: 19243282
Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study, PMID: 19652058
Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib, PMID: 19174554
Synergistic induction of apoptosis by mapatumumab and anthracyclines in human bladder cancer cells, PMID: 25483927
Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study, PMID: 19690193
A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma, PMID: 21081929
Phase II trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer, PMID: 24560012
A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma, PMID: 26802147
Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer, PMID: 20068564
A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies, PMID: 18519776
Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer, PMID: 18255187
Technology evaluation: mapatumumab, Human Genome Sciences/GlaxoSmithKline/Takeda, PMID: 16248286
Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1, PMID: 17416859
Human agonistic TRAIL receptor antibodies Mapatumumab and Lexatumumab induce apoptosis in malignant mesothelioma and act synergistically with cisplatin, PMID: 17953743
Altered regulation of extrinsic apoptosis pathway in HCV-infected HCC cells enhances susceptibility to mapatumumab-induced apoptosis, PMID: 19788693
Induction of potent NK cell-dependent anti-myeloma cytotoxic T cells in response to combined mapatumumab and bortezomib, PMID: 26405606
Augmented antitumor activity against B-cell lymphoma by a combination of monoclonal antibodies targeting TRAIL-R1 and CD20, PMID: 17671142
The role of Bcl-xL in synergistic induction of apoptosis by mapatumumab and oxaliplatin in combination with hyperthermia on human colon cancer, PMID: 23051936
Hyperthermia-enhanced TRAIL- and mapatumumab-induced apoptotic death is mediated through mitochondria in human colon cancer cells, PMID: 22174016
Hyperthermia enhances mapatumumab-induced apoptotic death through ubiquitin-mediated degradation of cellular FLIP(long) in human colon cancer cells, PMID: 23559011
Combination of the pro-apoptotic TRAIL-receptor antibody mapatumumab with ionizing radiation strongly increases long-term tumor control under ambient and hypoxic conditions, PMID: 19695436
Initial testing (stage 1) of mapatumumab (HGS-ETR1) by the pediatric preclinical testing program, PMID: 19856388
99m Tc-Labeled lexatumumab, PMID: 20641185
111 In-Labeled lexatumumab, PMID: 20641892
Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin, PMID: 27879498
Evidence for two modes of synergistic induction of apoptosis by mapatumumab and oxaliplatin in combination with hyperthermia in human colon cancer cells, PMID: 24013390
TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors, PMID: 20940278
[Molecular targets in colon cancer], PMID: 16689454
Translating TRAIL-receptor targeting agents to the clinic, PMID: 22531313
Restoring TRAIL mediated signaling in ovarian cancer cells, PMID: 25030086
TRAIL-based therapeutic approaches for the treatment of pediatric malignancies, PMID: 23458616
Human monoclonal antibodies in cancer therapy: a review of recent developments, PMID: 23276961
Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells, PMID: 22738917
Development of a radioiodinated apoptosis-inducing ligand, rhTRAIL, and a radiolabelled agonist TRAIL receptor antibody for clinical imaging studies, PMID: 22014269
Dacarbazine and the agonistic TRAIL receptor-2 antibody lexatumumab induce synergistic anticancer effects in melanoma, PMID: 23029050
Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells, PMID: 30947287
Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer, PMID: 21653692
Gateways to clinical trials, PMID: 17440629
Gateways to clinical trials, PMID: 16395422
Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma, PMID: 17335294
Gateways to clinical trials, PMID: 16810345
Gateways to clinical trials, PMID: 16845450
Gateways to clinical trials, PMID: 19088949
Primary ovarian cancer cells are sensitive to the proaptotic effects of proteasome inhibitors, PMID: 20126991
Gateways to clinical trials, PMID: 20664824
TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5, PMID: 20354842
The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials, PMID: 33791337
RIP1 is required for IAP inhibitor-mediated sensitization for TRAIL-induced apoptosis via a RIP1/FADD/caspase-8 cell death complex, PMID: 22890322
Carboxypeptidase A4 negatively regulates HGS-ETR1/2-induced pyroptosis by forming a positive feedback loop with the AKT signalling pathway., PMID:38049405
TRAIL-mediated signaling in bladder cancer: realization of clinical efficacy of TRAIL-based therapeutics in medical oncology., PMID:37432489
miR-3132 upregulates surface TRAIL to induce apoptotic cell death in cancer cells., PMID:35141020
The TRAIL in the Treatment of Human Cancer: An Update on Clinical Trials., PMID:33791337
Impact of p53 status on TRAIL-mediated apoptotic and non-apoptotic signaling in cancer cells., PMID:30947287
Transient stabilization, rather than inhibition, of MYC amplifies extrinsic apoptosis and therapeutic responses in refractory B-cell lymphoma., PMID:30914792
Enhancement of death receptor 4-mediated apoptosis and cytotoxicity in renal cell carcinoma cells by anisomycin., PMID:27879498
A randomized, double-blind, placebo-controlled phase II study to assess the efficacy and safety of mapatumumab with sorafenib in patients with advanced hepatocellular carcinoma., PMID:26802147
Induction of potent NK cell-dependent anti-myeloma cytotoxic T cells in response to combined mapatumumab and bortezomib., PMID:26405606
Synergistic induction of apoptosis by mapatumumab and anthracyclines in human bladder cancer cells., PMID:25483927
Restoring TRAIL mediated signaling in ovarian cancer cells., PMID:25030086
Phase II trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer., PMID:24560012
Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis., PMID:24086526
Evidence for two modes of synergistic induction of apoptosis by mapatumumab and oxaliplatin in combination with hyperthermia in human colon cancer cells., PMID:24013390
Hyperthermia enhances mapatumumab-induced apoptotic death through ubiquitin-mediated degradation of cellular FLIP(long) in human colon cancer cells., PMID:23559011
TRAIL-based therapeutic approaches for the treatment of pediatric malignancies., PMID:23458616
Human monoclonal antibodies in cancer therapy: a review of recent developments., PMID:23276961
The role of Bcl-xL in synergistic induction of apoptosis by mapatumumab and oxaliplatin in combination with hyperthermia on human colon cancer., PMID:23051936
Dacarbazine and the agonistic TRAIL receptor-2 antibody lexatumumab induce synergistic anticancer effects in melanoma., PMID:23029050
RIP1 protein-dependent assembly of a cytosolic cell death complex is required for inhibitor of apoptosis (IAP) inhibitor-mediated sensitization to lexatumumab-induced apoptosis., PMID:22927431
Mitomycin C potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: evidence for the role of c-Jun N-terminal kinase activation., PMID:22895172
RIP1 is required for IAP inhibitor-mediated sensitization for TRAIL-induced apoptosis via a RIP1/FADD/caspase-8 cell death complex., PMID:22890322
Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells., PMID:22738917
Translating TRAIL-receptor targeting agents to the clinic., PMID:22531313
Hyperthermia-enhanced TRAIL- and mapatumumab-induced apoptotic death is mediated through mitochondria in human colon cancer cells., PMID:22174016
Development of a radioiodinated apoptosis-inducing ligand, rhTRAIL, and a radiolabelled agonist TRAIL receptor antibody for clinical imaging studies., PMID:22014269
Prediction of proapoptotic anticancer therapeutic response in vivo based on cell death visualization and TRAIL death ligand-receptor interaction., PMID:21785270
Death receptor 4 is preferentially recruited to lipid rafts in chronic lymphocytic leukemia cells contributing to tumor necrosis related apoptosis inducing ligand-induced synergistic apoptotic responses., PMID:21699383
Off-target lapatinib activity sensitizes colon cancer cells through TRAIL death receptor up-regulation., PMID:21653830
Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer., PMID:21653692
High TRAIL-R3 expression on leukemic blasts is associated with poor outcome and induces apoptosis-resistance which can be overcome by targeting TRAIL-R2., PMID:21281967
A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma., PMID:21081929
TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors., PMID:20940278
Gateways to clinical trials., PMID:20664824
TRAIL signaling is mediated by DR4 in pancreatic tumor cells despite the expression of functional DR5., PMID:20354842
28th Annual JPMorgan Healthcare Conference--Human Genome Sciences and Celgene., PMID:20191423
Primary ovarian cancer cells are sensitive to the proaptotic effects of proteasome inhibitors., PMID:20126991
Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer., PMID:20068564
Initial testing (stage 1) of mapatumumab (HGS-ETR1) by the pediatric preclinical testing program., PMID:19856388
Altered regulation of extrinsic apoptosis pathway in HCV-infected HCC cells enhances susceptibility to mapatumumab-induced apoptosis., PMID:19788693
Phosphorothioate-modified TLR9 ligands protect cancer cells against TRAIL-induced apoptosis., PMID:19734228
Combination of the pro-apoptotic TRAIL-receptor antibody mapatumumab with ionizing radiation strongly increases long-term tumor control under ambient and hypoxic conditions., PMID:19695436
Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study., PMID:19690193
Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study., PMID:19652058
Efficacy of triple therapies including ionising radiation, agonistic TRAIL antibodies and cisplatin., PMID:19424623
p65 activity and ZAP-70 status predict the sensitivity of chronic lymphocytic leukemia cells to the selective IkappaB kinase inhibitor BMS-345541., PMID:19351760