Catalog No.
DHJ61001
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
Endosialin, TEM1, CD164L1, Tumor endothelial marker 1, CD248
Concentration
1.1 mg/ml
Endotoxin level
Please contact with the lab for this information.
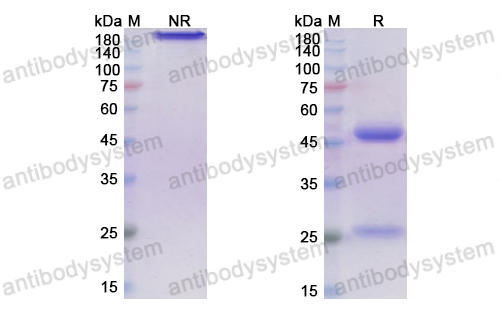
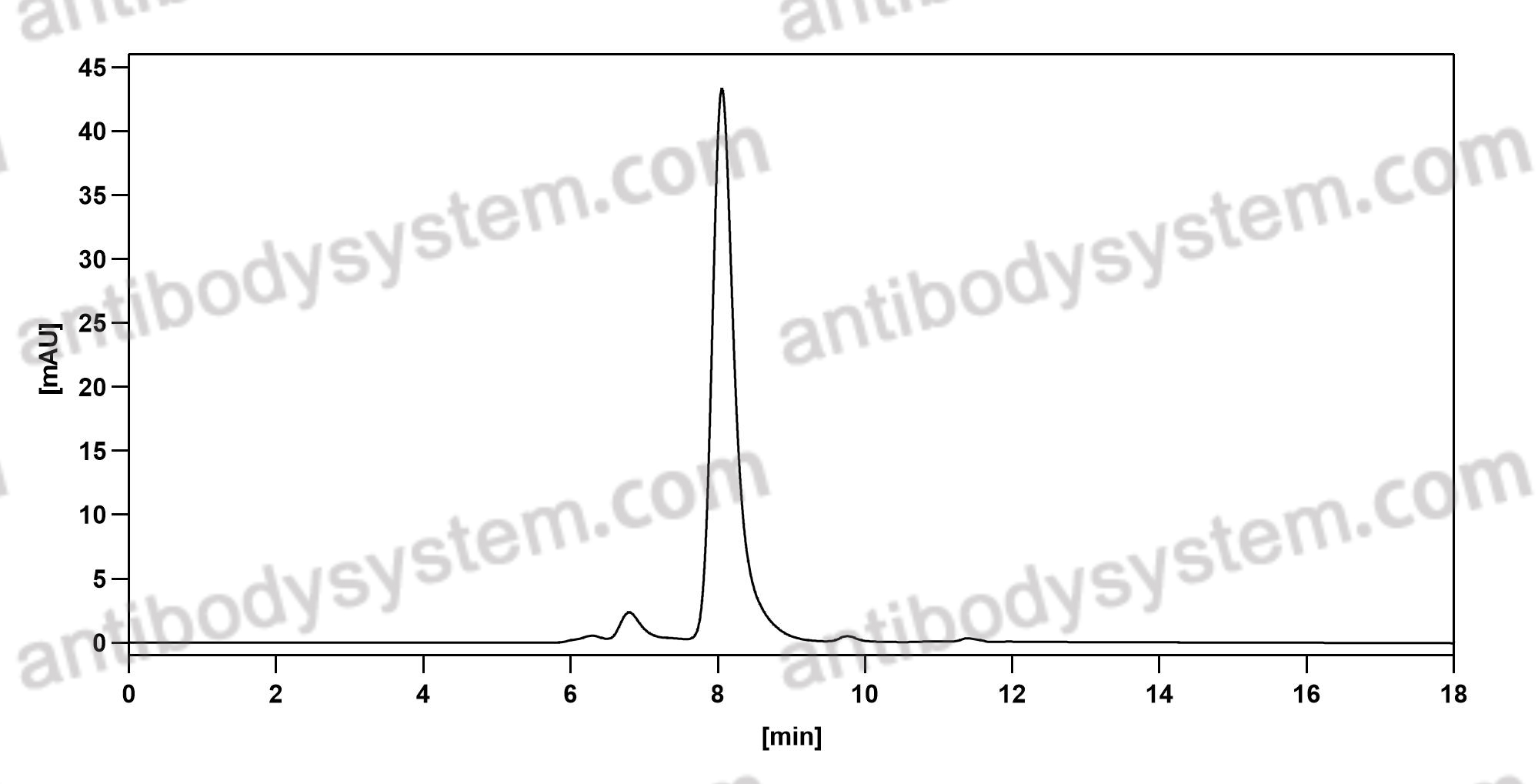
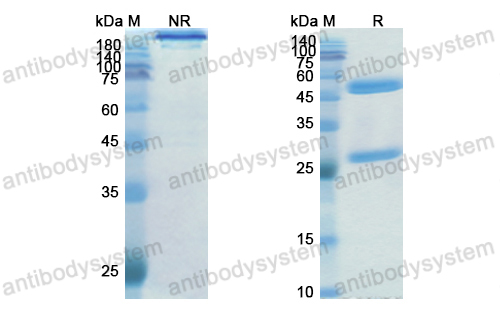
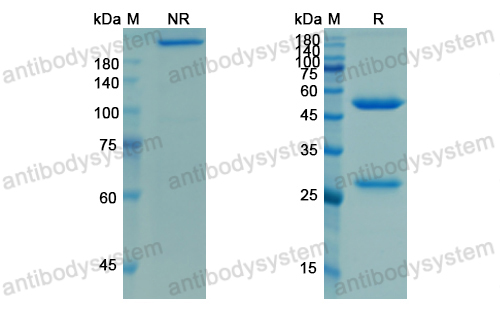
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
Q9HCU0
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
MORAb-004, CAS: 946415-62-9
Clone ID
Ontuxizumab
Development of 89Zr-Ontuxizumab for in vivo TEM-1/endosialin PET applications, PMID: 26909615
Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: A report from the Children's Oncology Group Phase 1 Pilot Consortium (ADVL1213), PMID: 29292843
A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas, PMID: 31034598
A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic melanoma, PMID: 29127533
A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors, PMID: 30623276
A Randomized, Double-Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Monotherapy Ontuxizumab (MORAb-004) Plus Best Supportive Care in Patients with Chemorefractory Metastatic Colorectal Cancer, PMID: 29084918
CD248 as a novel therapeutic target in pulmonary arterial hypertension, PMID: 32997414
Novel antibody probes for the characterization of endosialin/TEM-1, PMID: 27494870
Endosialin Expression in Metastatic Melanoma Tumor Microenvironment Vasculature: Potential Therapeutic Implications, PMID: 26085332
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies, PMID: 33807678
Antibody-based tumor vascular theranostics targeting endosialin/TEM1 in a new mouse tumor vascular model, PMID: 24553243
Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature, PMID: 26327620
A first-in-human phase I study of MORAb-004, a monoclonal antibody to endosialin in patients with advanced solid tumors, PMID: 25398449
TEM1/endosialin/CD248 promotes pathologic scarring and TGF-β activity through its receptor stability in dermal fibroblasts., PMID:38254097
An individualized stemness-related signature to predict prognosis and immunotherapy responses for gastric cancer using single-cell and bulk tissue transcriptomes., PMID:38168907
Endosialin/CD248 may be a potential therapeutic target to prevent the invasion and metastasis in osteosarcoma., PMID:34976154
Invasive Fungal Diseases in Children with Hematological Malignancies Treated with Therapies That Target Cell Surface Antigens: Monoclonal Antibodies, Immune Checkpoint Inhibitors and CAR T-Cell Therapies., PMID:33807678
CD248 as a novel therapeutic target in pulmonary arterial hypertension., PMID:32997414
A phase 1 and randomized controlled phase 2 trial of the safety and efficacy of the combination of gemcitabine and docetaxel with ontuxizumab (MORAb-004) in metastatic soft-tissue sarcomas., PMID:31034598
A phase I study of ontuxizumab, a humanized monoclonal antibody targeting endosialin, in Japanese patients with solid tumors., PMID:30623276
Phase 1 trial of ontuxizumab (MORAb-004) in children with relapsed or refractory solid tumors: A report from the Children's Oncology Group Phase 1 Pilot Consortium (ADVL1213)., PMID:29292843
A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic melanoma., PMID:29127533
A Randomized, Double-Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Monotherapy Ontuxizumab (MORAb-004) Plus Best Supportive Care in Patients with Chemorefractory Metastatic Colorectal Cancer., PMID:29084918
Novel antibody probes for the characterization of endosialin/TEM-1., PMID:27494870
Development of 89Zr-Ontuxizumab for in vivo TEM-1/endosialin PET applications., PMID:26909615
Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature., PMID:26327620
Endosialin Expression in Metastatic Melanoma Tumor Microenvironment Vasculature: Potential Therapeutic Implications., PMID:26085332
A first-in-human phase I study of MORAb-004, a monoclonal antibody to endosialin in patients with advanced solid tumors., PMID:25398449
Antibody-based tumor vascular theranostics targeting endosialin/TEM1 in a new mouse tumor vascular model., PMID:24553243