Catalog No.
KVV99903
Description
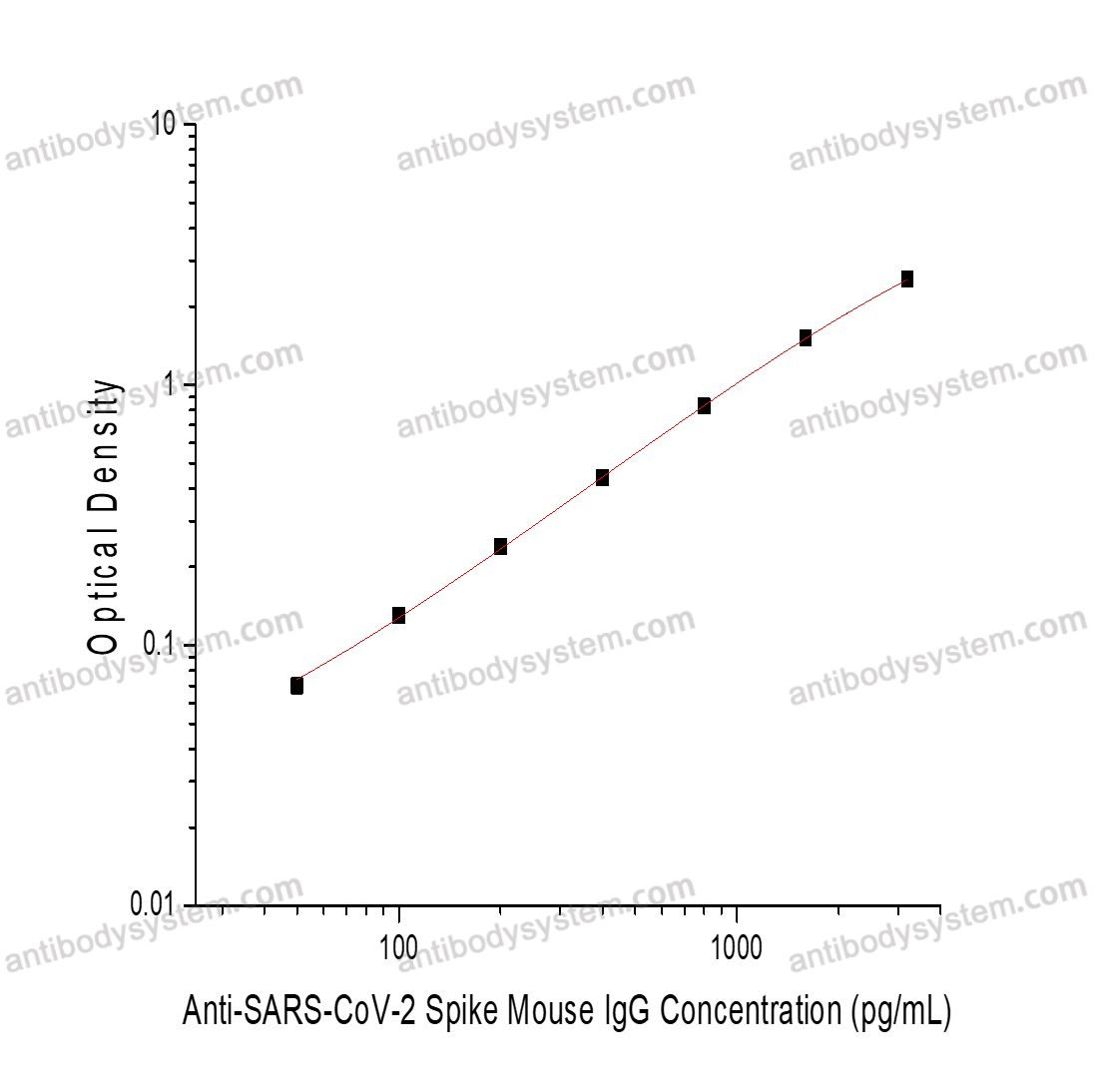
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. SARS-CoV-2 Spike has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Spike Mouse IgG present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Goat Anti-Mouse IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Spike Mouse IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Spike Mouse IgG concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative (Competitive)
Range
50 - 3,200 pg/mL
Sensitivity
25.23 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (pg/mL)
|
1463.2
|
410.2
|
186.0
|
1410.7
|
372.1
|
110.1
|
|
Standard deviation
|
84.9
|
17.6
|
6.3
|
68.4
|
16.9
|
11.3
|
|
CV (%)
|
5.8
|
4.3
|
3.4
|
4.9
|
4.6
|
10.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
Spike glycoprotein, S glycoprotein
Immunologic responses to an extracellular vesicle-based vaccine expressing the full suite of SARS-CoV-2 structural proteins., PMID:40532478
Genetic markers of enhanced functional antibody responses to COVID-19 vaccination., PMID:40527060
Negative associations of age and lifestyle factors with the antibody response to the COVID-19 vaccine BNT162b2 in health workers from Spain., PMID:40519918
Characteristics of Kawasaki disease in children with a history of COVID-19., PMID:40517900
Covalent Functionalization of Layered Double Hydroxides to Generate Peptide-Based SARS-CoV-2 Nanovaccine., PMID:40508447
Development and Validation of Novel Cell-free Direct Neutralization Assay for SARS-CoV-2., PMID:40499786
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection., PMID:40498720
Determination of resilience of a panel of broadly neutralizing mAbs to emerging variants of SARS-CoV-2 generated using reverse genetics., PMID:40496810
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2., PMID:40488473
Immune age is correlated with decreased TCR clonal diversity and antibody response to SARS-CoV-2., PMID:40481111
Safety and immunogenicity of four sequential doses of NVX-CoV2373 in adults and adolescents: A phase 3, randomized, placebo-controlled trial (PREVENT-19)., PMID:40479932
Development and Concordance of Binding and Neutralizing Assays to Determine SARS-CoV-2 Antibody Activity in Human Milk., PMID:40475854
Serum Levels of IL-21 and IL-27 Do not Reflect differential Avidity of Anti-SARS-CoV-2 IgG Antibodies in Symptomatic and Asymptomatic COVID-19 Patients., PMID:40471646
GWAS identifies genetic loci for antibody response to SARS-CoV-2 vaccines in patients with systemic autoimmune diseases and healthy individuals., PMID:40463545
Tracking the dynamics of antibody production against the SARS-CoV-2 virus after two doses of the mRNA vaccine BNT162b2., PMID:40461246
Adults in Ghana generate higher and more durable neutralising antibody titres following primary course COVID-19 vaccination than matched UK adults: The HERITAGE Study., PMID:40437463
Systems serology-based comparison of humoral immune responses induced by liposome or aluminum hydroxide adjuvanted SARS-CoV-2 spike protein., PMID:40436929
A Luciferase-Based Approach for Functional Screening of 5' and 3' Untranslated Regions of the mRNA Component for mRNA Vaccines., PMID:40432139
Serological Correlate of Protection Established by Neutralizing Antibodies Differs Among Dialysis Patients with SARS-CoV-2 Variants of Concern., PMID:40432127
SARS-CoV-2 Antibodies in Response to COVID-19 Vaccination in Underserved Racial/Ethnic Minority People Living with HIV., PMID:40432125
Vaccine-Induced Humoral and Cellular Response to SARS-CoV-2 in Multiple Sclerosis Patients on Ocrelizumab., PMID:40432100
Antibody Response Against SARS-CoV-2 Spike Protein in People with HIV After COVID-19 Vaccination., PMID:40432092
Long-Term Dynamics of SARS-CoV-2 Variant-Specific Neutralizing Antibodies Following mRNA Vaccination and Infection., PMID:40431687
Impact of Vaccine-Elicited Anti-Spike IgG4 Antibodies on Fc-Effector Functions Against SARS-CoV-2., PMID:40431678
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Evaluating SARS-CoV-2 T Cell Immunity in COVID-19-Naive Vaccinated Individuals with and Without Spike Protein IgG Antibodies., PMID:40430736
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
A combined digital microfluidic test for assessing infection and immunity status for viral disease in saliva., PMID:40423685
Immunogenicity of COVID-19 Vaccination in Immunocompromised Patients (Auto-COVID-VACC): Protocol for Multicenter Prospective Noninterventional Study., PMID:40418566
mRNA COVID-19 vaccines induce superior immunoglobulin A titers in patients with cancer compared with viral vector vaccines: implications for immunization strategies., PMID:40414552
Comparative study of humoral and cellular immunity against SARS-CoV-2 induced by different COVID-19 vaccine types: Insights into protection against wildtype, Delta and JN.1 omicron strains., PMID:40408899
Evaluation of humoral and cellular immune responses in healthcare workers with varying levels of SARS-CoV-2 exposure: effects of CoronaVac vaccination followed by heterologous booster., PMID:40406109
Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection., PMID:40406038
Specific thresholds of circulating antibody titers predict against infection and reduced disease severity in SARS-CoV-2 close contacts., PMID:40405410
The Combination of TLR4 and TLR9 Agonists with Self-Amplifying RNA Lipid Nanoparticles Leads to a More Powerful Immune Response Against SARS-CoV-2., PMID:40401447
Virus-specific antibody responses in multiple sclerosis patients treated with Ocrevus., PMID:40398376
Hybrid B- and T-Cell Immunity Associates With Protection Against Breakthrough Infection After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination in Avon Longitudinal Study of Parents and Children (ALSPAC) Participants., PMID:40392230
Longitudinal assessment of COVID-19 vaccine immunogenicity in people with HIV stratified by CD4+ T-cell count in the Netherlands: A two-year follow-up study., PMID:40388467
Analysis of humoral and cellular immune activation up to 21 months after heterologous and homologous COVID-19 vaccination., PMID:40386778
Immunogenicity Evaluation of a SARS-CoV-2 BA.2 Subunit Vaccine Formulated with CpG 1826 plus alum Dual Adjuvant., PMID:40383947
An RBD-Fc mucosal vaccine provides variant-proof protection against SARS-CoV-2 in mice and hamsters., PMID:40383816
Analysis of the Total Immunoglobulin G (IgG) and Its Subclasses Over Time in Coronavirus Disease 2019-Recovered Patients and Its Association With Disease Severity: A Single-Center Prospective Cohort Study., PMID:40383689
Immune imprinting and vaccine interval determine antibody responses to monovalent XBB.1.5 COVID-19 vaccination., PMID:40382525
Estimating population immunity to SARS-CoV-2 by random sampling from primary and secondary healthcare in Scotland, May 2024., PMID:40381379
Safety and immunogenicity of SARS-CoV-2 protein subunit recombinant vaccine (Indovac®) in healthy populations aged 18 years and above in Indonesia: A phase I, observer-blind, randomized, controlled study., PMID:40381203
Comparative performance of serum and plasma samples in SARS-CoV-2 serology and neutralization assays., PMID:40374015
Dynamics of SARS-CoV-2 Immunoglobulin G Antibody Among Hospitalized Patients and Healthcare Workers During the Delta Wave in Bangladesh., PMID:40370886
Vaccine-Induced Specific Cellular and Humoral Immunity after MRNA-Based COVID-19 Vaccination in Athletes and Controls., PMID:40367509
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity., PMID:40359129
Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals., PMID:40358138