Catalog No.
DHD14205
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
PEMT, CA 15-3, H23AG, Peanut-reactive urinary mucin, PEM, EMA, Cancer antigen 15-3, MUC-1, Episialin, MUC1-CT, MUC1-beta, Tumor-associated mucin, CD227, Polymorphic epithelial mucin, KL-6, MUC1-alpha, MUC1, PUM, Krebs von den Lungen-6, MUC1-NT, Tumor-associated epithelial membrane antigen, Mucin-1, Breast carcinoma-associated antigen DF3, Carcinoma-associated mucin
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
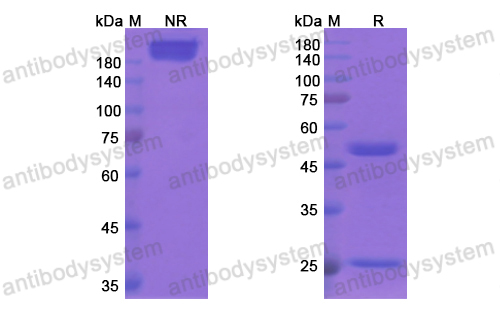
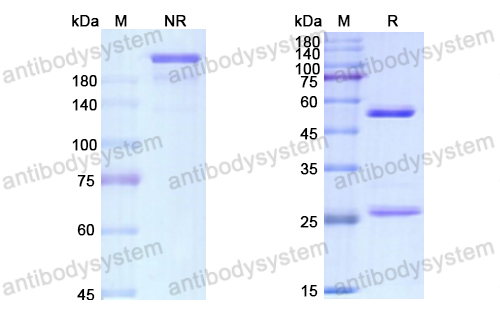
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P15941
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
IMMU-107, hPAM4, hPAM4 IgG-DOTA, hPAM4-DOTA, CAS: 1622075-09-5
Clone ID
Clivatuzumab
(90)Y-clivatuzumab tetraxetan with or without low-dose gemcitabine: A phase Ib study in patients with metastatic pancreatic cancer after two or more prior therapies, PMID: 26187510
Identification of PAM4 (clivatuzumab)-reactive epitope on MUC5AC: a promising biomarker and therapeutic target for pancreatic cancer, PMID: 25595893
Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial, PMID: 22569804
Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: a phase I single-dose escalation trial, PMID: 21527562
Mapping PAM4 (clivatuzumab), a monoclonal antibody in clinical trials for early detection and therapy of pancreatic ductal adenocarcinoma, to MUC5AC mucin, PMID: 24257318
Antibodies to watch in 2015, PMID: 25484055
The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy, PMID: 24818166
Antibodies to watch in 2014: mid-year update, PMID: 24846335
Targeted radionuclide therapies for pancreatic cancer, PMID: 26227823
Society of Nuclear Medicine--57th annual meeting, PMID: 20721816
Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine, PMID: 19949026
Combination radioimmunotherapy and chemoimmunotherapy involving different or the same targets improves therapy of human pancreatic carcinoma xenograft models, PMID: 21467164
Targeted radionuclide therapies for pancreatic cancer., PMID:26227823
(90)Y-clivatuzumab tetraxetan with or without low-dose gemcitabine: A phase Ib study in patients with metastatic pancreatic cancer after two or more prior therapies., PMID:26187510
Identification of PAM4 (clivatuzumab)-reactive epitope on MUC5AC: a promising biomarker and therapeutic target for pancreatic cancer., PMID:25595893
Antibodies to watch in 2015., PMID:25484055
Antibodies to watch in 2014: mid-year update., PMID:24846335
The role of PAM4 in the management of pancreatic cancer: diagnosis, radioimmunodetection, and radioimmunotherapy., PMID:24818166
Mapping PAM4 (clivatuzumab), a monoclonal antibody in clinical trials for early detection and therapy of pancreatic ductal adenocarcinoma, to MUC5AC mucin., PMID:24257318
Fractionated radioimmunotherapy with (90) Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial., PMID:22569804
Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: a phase I single-dose escalation trial., PMID:21527562
Combination radioimmunotherapy and chemoimmunotherapy involving different or the same targets improves therapy of human pancreatic carcinoma xenograft models., PMID:21467164
Society of Nuclear Medicine--57th annual meeting., PMID:20721816
Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine., PMID:19949026