Catalog No.
VVV24006
Species reactivity
Influenza A virus (A/Brisbane/59-MA/2007(H1N1))(A0A2D3I2L6), Influenza A virus (strain swl A/California/04/2009 H1N1) (A0A0C4ZKQ4), (P03468) Influenza A virus (strain A/Puerto Rico/8/1934 H1N1), Influenza A virus (strain A/Vietnam/1203/2004 H5N1) (Q6DPL2), Influenza A virus (strain A/Hong Kong/1/1968 H3N2) (Q910S0), Influenza A virus (A/Fujian/411/2002(H3N2)) (H8P392), Influenza B virus (strain B/Lee/1940) (P03474), Influenza B virus (B/Wisconsin/01/2010) (G8JJM2), Influenza B virus (B/Brisbane/60/2008) (C0LT34), Influenza B virus (B/Texas/02/2013) (R9WY37), Influenza B virus (B/Colorado/06/2017) (A0A1X9RX69)
Host species
Human
Isotype
IgG1, kappa
Clonality
Monoclonal
Target
NA, Neuraminidase
Concentration
2.68 mg/ml
Endotoxin level
Please contact with the lab for this information.
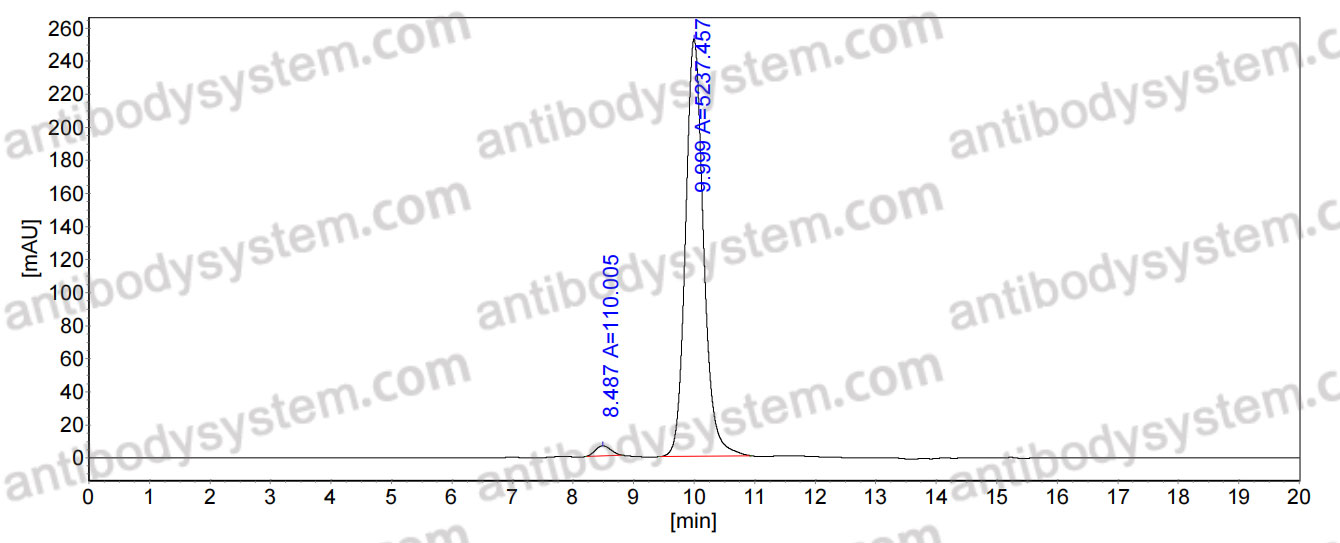
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
A0A2D3I2L6, A0A0C4ZKQ4, P03468, Q6DPL2, Q910S0, H8P392, P03474, G8JJM2, C0LT34, R9WY37, A0A1X9RX69
Applications
ELISA, Neutralization
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Clone ID
Iv0283
A monoclonal anti-hemagglutinin stem antibody modified with zanamivir protects against both influenza A and B viruses., PMID:40193611
Broadly protective bispecific antibodies that simultaneously target influenza virus hemagglutinin and neuraminidase., PMID:38899870
Fc-Effector-Independent in vivo Activity of a Potent Influenza B Neuraminidase Broadly Neutralizing Antibody., PMID:37515226
Highly Cross-Reactive and Protective Influenza A Virus H3N2 Hemagglutinin- and Neuraminidase-Specific Human Monoclonal Antibodies., PMID:37318331
Multi-Influenza HA Subtype Protection of Ferrets Vaccinated with an N1 COBRA-Based Neuraminidase., PMID:36680224
A broadly protective human monoclonal antibody targeting the sialidase activity of influenza A and B virus neuraminidases., PMID:36329075
Characterization of Immune Response towards Generation of Universal Anti-HA-Stalk Antibodies after Immunization of Broiler Hens with Triple H5N1/NA-HA-M1 VLPs., PMID:35458460
Supplementation of H7N9 Virus-Like Particle Vaccine With Recombinant Epitope Antigen Confers Full Protection Against Antigenically Divergent H7N9 Virus in Chickens., PMID:35265069
Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains., PMID:34160258
Structure-Based Modification of an Anti-neuraminidase Human Antibody Restores Protection Efficacy against the Drifted Influenza Virus., PMID:33024040
Broadly protective human antibodies that target the active site of influenza virus neuraminidase., PMID:31649200
Elicitation of Broadly Protective Antibodies following Infection with Influenza Viruses Expressing H1N1 Computationally Optimized Broadly Reactive Hemagglutinin Antigens., PMID:31022693
Enhanced cross-lineage protection induced by recombinant H9N2 avian influenza virus inactivated vaccine., PMID:30797637
Highly immunogenic influenza virus-like particles containing B-cell-activating factor (BAFF) for multi-subtype vaccine development., PMID:30738089
Antibody Determinants of Influenza Immunity., PMID:30715373
Neuraminidase inhibition contributes to influenza A virus neutralization by anti-hemagglutinin stem antibodies., PMID:30683737
Immunodominance and Antigenic Variation of Influenza Virus Hemagglutinin: Implications for Design of Universal Vaccine Immunogens., PMID:30535315
Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody., PMID:30381484
Computational screening of known broad-spectrum antiviral small organic molecules for potential influenza HA stem inhibitors., PMID:30180218
Intra-seasonal antibody repertoire analysis of a subject immunized with an MF59®-adjuvanted pandemic 2009 H1N1 vaccine., PMID:30055967
Immunogenicity and Protection Against Influenza H7N3 in Mice by Modified Vaccinia Virus Ankara Vectors Expressing Influenza Virus Hemagglutinin or Neuraminidase., PMID:29599502
Development of Clade-Specific and Broadly Reactive Live Attenuated Influenza Virus Vaccines against Rapidly Evolving H5 Subtype Viruses., PMID:28490598
Nanostructured glycan architecture is important in the inhibition of influenza A virus infection., PMID:27775724
Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection., PMID:26731473
Protection against H5N1 Influenza Virus Induced by Matrix-M Adjuvanted Seasonal Virosomal Vaccine in Mice Requires Both Antibodies and T Cells., PMID:26696245
Progress on adenovirus-vectored universal influenza vaccines., PMID:25876176
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice., PMID:24920793
An influenza N1 neuraminidase-specific monoclonal antibody with broad neuraminidase inhibition activity against H5N1 HPAI viruses., PMID:21922687
Influenza vaccine immunology., PMID:21198671
A pandemic influenza H1N1 live vaccine based on modified vaccinia Ankara is highly immunogenic and protects mice in active and passive immunizations., PMID:20808939
An enzymatic virus-like particle assay for sensitive detection of virus entry., PMID:19879300