Anti-CGRP monoclonal antibodies counteract establishment of food aversive memories and chemotherapy-induced anorexia and weight loss., PMID:40490094
Pharmacovigilance analysis of neurological adverse events associated with GLP-1 receptor agonists based on the FDA Adverse Event Reporting System., PMID:40413246
Effects of GLP-1 Analogues and Agonists on the Gut Microbiota: A Systematic Review., PMID:40284168
Comparative efficacy and tolerability of currently approved incretin mimetics: A systematic analysis of placebo-controlled clinical trials., PMID:40212008
Influence of Combination Therapy with Dulaglutide or Liraglutide and SGLT2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Post-hoc Analysis of the RECAP Study., PMID:40105226
Exploring Connections Between Weight-Loss Medications and Thyroid Cancer: A Look at the FDA Adverse Event Reporting System Database., PMID:40055991
Sex Differences in the Efficacy of Glucagon-Like Peptide-1 Receptor Agonists for Weight Reduction: A Systematic Review and Meta-Analysis., PMID:40040445
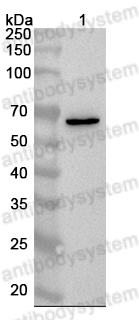
Dulaglutide accelerates diabetic wound healing by suppressing Nrf2-dependent ferroptosis in diabetic mice., PMID:39954860
Improvement in Helicobacter pylori Eradication Among Adults Receiving Semaglutide: A Population-Based Propensity-Score-Adjusted Analysis., PMID:39902748
Association between glucagon-like peptide-1 agonists and risk of diabetic retinopathy: a disproportionality analysis using FDA adverse event reporting system data., PMID:39873334
Selective Colocalization of GHSR and GLP-1R in a Subset of Hypothalamic Neurons and Their Functional Interaction., PMID:39737802
Differential effects of liraglutide naltrexone/bupropion, and caloric restriction on metabolic parameters and beta-cell regeneration in type 2 diabetic rat model: role of beta arrestin 1., PMID:39704859
The Cardiovascular Outcomes Between Liraglutide and Dulaglutide Among Different Chronic Kidney Disease Stages in Patients With Type 2 Diabetes., PMID:39689782
Comparative cardiovascular and renal outcomes of Liraglutide versus Dulaglutide in Asian type 2 diabetes patients., PMID:39528690
Utilising Health Technology Assessment to Develop Managed Access Protocols to Facilitate Drug Reimbursement in Ireland., PMID:39133443
Efficacy, adherence and persistence of various glucagon-like peptide-1 agonists: nationwide real-life data., PMID:39109455
The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in type 2 diabetes patients (TROPHIES): resource use and costs of treatment in clinical practice in France, Germany, and Italy., PMID:38963346
Cutaneous hypersensitivity reaction to dulaglutide: A case report of an allergic reaction., PMID:38942076
Adverse event comparison between glucagon-like peptide-1 receptor agonists and other antiobesity medications following bariatric surgery., PMID:38934217
Effects of glucagon-like peptide-1 receptor agonists on gastric mucosal visibility and retained gastric contents during EGD., PMID:38759761
Safe Continuation of Glucagon-like Peptide 1 Receptor Agonists at Endoscopy: A Case Series of 57 Adults Undergoing Endoscopic Sleeve Gastroplasty., PMID:38753265
Comparative Analysis of the Anti-Inflammatory Effects of Liraglutide and Dulaglutide., PMID:38749748
Switching to Tirzepatide 5 mg From Glucagon-Like Peptide-1 Receptor Agonists: Clinical Expectations in the First 12 Weeks of Treatment., PMID:38723893
Effects of glucagon-like peptide-1 receptor agonists on upper endoscopy in diabetic and nondiabetic patients., PMID:38692518
Existing and emerging GLP-1 receptor agonist therapy: Ramifications for diabetic retinopathy screening., PMID:38578067
Effects of GLP-1 receptor agonists on the degree of liver fibrosis and CRP in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis: A systematic review and meta-analysis., PMID:38555202
Glucagon-like peptide-1 receptor agonists modestly reduced blood pressure among patients with and without diabetes mellitus: A meta-analysis and meta-regression., PMID:38505997
An oncolytic vaccinia virus encoding hyaluronidase reshapes the extracellular matrix to enhance cancer chemotherapy and immunotherapy., PMID:38458640
Safety and Efficacy of Efruxifermin in Combination With a GLP-1 Receptor Agonist in Patients With NASH/MASH and Type 2 Diabetes in a Randomized Phase 2 Study., PMID:38447814
The role of glucagon-like peptide 1 receptor agonists for weight control in individuals with acquired hypothalamic obesity-A systematic review., PMID:38273176
Leptin Reduction as a Required Component for Weight Loss., PMID:37935033
The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide and Liraglutide in Type 2 Diabetes Patients (TROPHIES): Final patient-reported outcomes at 24 months., PMID:37712754
Efficacy and Safety of a Biosimilar Liraglutide (Melitide®) Versus the Reference Liraglutide (Victoza®) in People with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Noninferiority Clinical Trial., PMID:37707701
The real-world observational prospective study of health outcomes with dulaglutide and liraglutide in patients with type 2 diabetes (TROPHIES): Final, 24-month analysis of time to first significant treatment change, treatment persistence and clinical outcomes., PMID:37700627
Maturity-onset diabetes of the young type 9 or latent autoimmune diabetes in adults: A case report and review of literature., PMID:37547587
Improvement of glycaemic control and treatment satisfaction by switching from liraglutide or dulaglutide to subcutaneous semaglutide in patients with type 2 diabetes: A multicentre, prospective, randomized, open-label, parallel-group comparison study (SWITCH-SEMA 1 study)., PMID:36722623
An update on peptide-based therapies for type 2 diabetes and obesity., PMID:36608818
The anti-inflammatory feature of glucagon-like peptide-1 and its based diabetes drugs-Therapeutic potential exploration in lung injury., PMID:36386481
Anti-diabetic effects of GLP1 analogs are mediated by thermogenic interleukin-6 signaling in adipocytes., PMID:36384099
Diabetes of Unclear Type in an Adolescent Boy With Multiple Islet-cell Autoantibody Positivity Successfully Managed With Glucagon-like Peptide-1 Receptor Agonist Alone: A Case Report., PMID:36075851
The Real-World Observational Prospective Study of Health Outcomes with Dulaglutide and Liraglutide in Patients with Type 2 Diabetes (TROPHIES): Patient disposition, clinical characteristics and treatment persistence at 12 months., PMID:35876235
A phase I study comparing the pharmacokinetics of the biosimilar (RD12014) with liraglutide (Victoza) in healthy Chinese male subjects., PMID:35871497
INnoVative trial design for testing the Efficacy, Safety and Tolerability of 6-month treatment with incretin-based therapy to prevent type 1 DIAbetes in autoantibody positive participants: A protocol for three parallel double-blind, randomised controlled trials (INVESTDIA)., PMID:35797241
Real-world effectiveness of liraglutide versus dulaglutide in Japanese patients with type 2 diabetes: a retrospective study., PMID:34997102
Combination of GLP-1 Receptor Activation and Glucagon Blockage Promotes Pancreatic β-Cell Regeneration In Situ in Type 1 Diabetic Mice., PMID:39280767
Lifetime Cost-effectiveness of Oral Semaglutide Versus Dulaglutide and Liraglutide in Patients With Type 2 Diabetes Inadequately Controlled With Oral Antidiabetics., PMID:34728099
Effects of GLP-1 receptor agonists on myokine levels and pro-inflammatory cytokines in patients with type 2 diabetes mellitus., PMID:34518091
NMR Spectroscopy for Protein Higher Order Structure Similarity Assessment in Formulated Drug Products., PMID:34299526
Association of IL-17 Inhibitor and SGLT2 Inhibitor with Candida Pyelonephritis., PMID:34270991
Effects of glucagon-like peptide-1 receptor agonists on secretions of insulin and glucagon and gastric emptying in Japanese individuals with type 2 diabetes: A prospective, observational study., PMID:34022121