Catalog No.
KDB54001
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
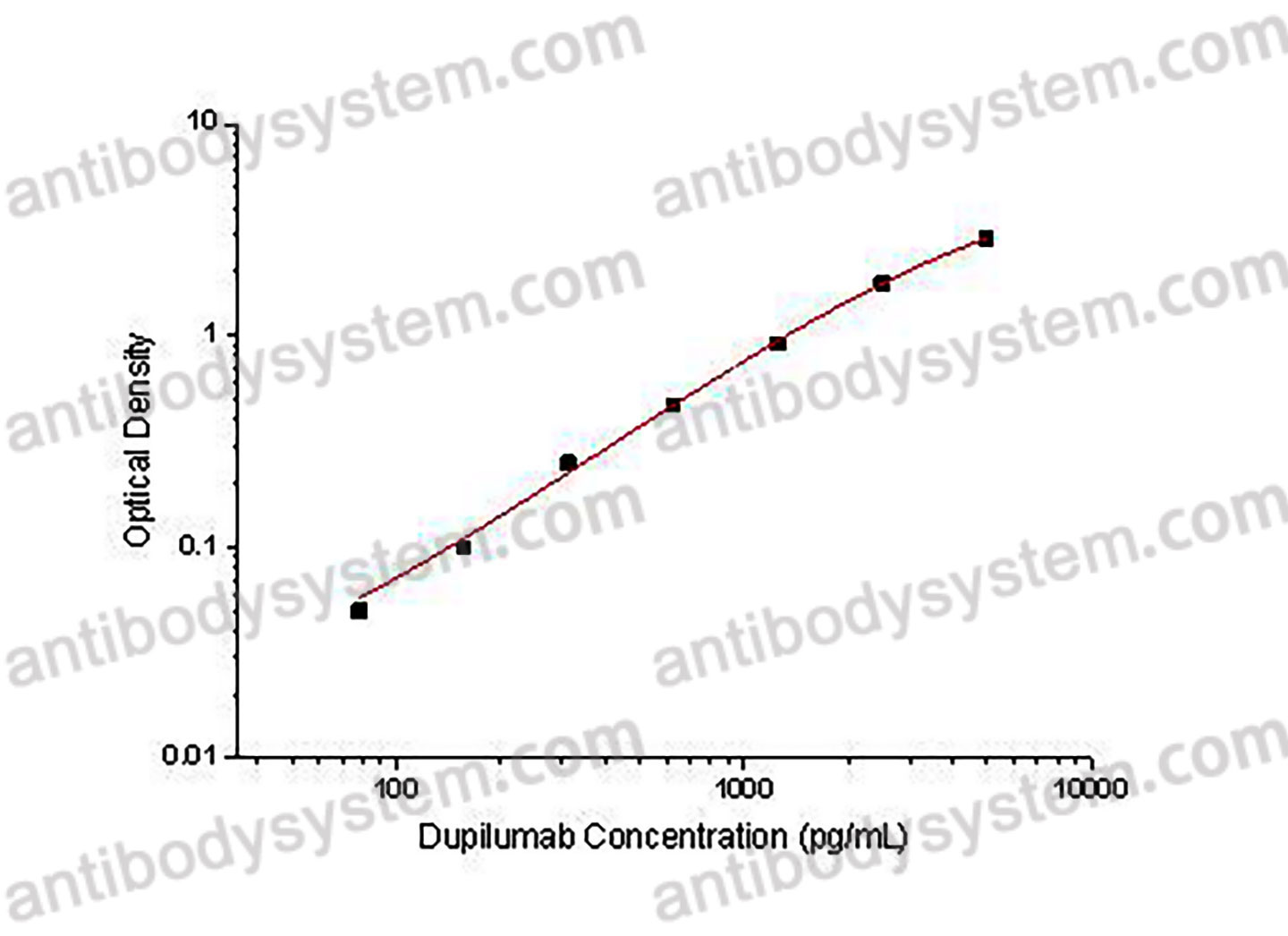
78.125 - 5,000 ng/mL
Sensitivity
33.12 ng/mL
Precision
CV<20%
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Alternative Names
PRA023, PRA-023, CAS: 2648504-55-4
Comparative Efficacy of Different Targeted Therapies in Patients With Moderate-to-Severe Ulcerative Colitis: Systematic Review/Network Meta-Analysis and Mechanistic Overview., PMID:40468857
Safety and efficacy of the anti-TL1A monoclonal antibody tulisokibart for Crohn's disease: a phase 2a induction trial., PMID:40456235
Tulisokibart shows promise for Crohn's disease., PMID:40456234
[Intestinal fibrosis in Crohn's disease : towards new therapeutic options?]., PMID:40079288
TL1A, a novel alarmin in airway, intestinal, and autoimmune disorders., PMID:40010414
TL1A: A model for a precision medicine approach in the treatment of Crohn's disease and ulcerative colitis., PMID:39521604
Phase 2 Trial of Anti-TL1A Monoclonal Antibody Tulisokibart for Ulcerative Colitis., PMID:39321363