Catalog No.
KDC43302
Description
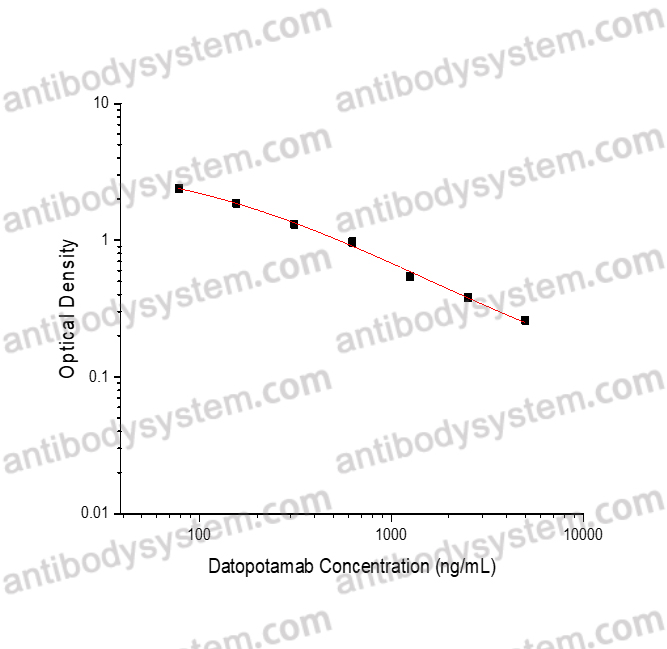
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human TACSTD2 has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Datopotamab in the sample competitively binds to the pre-coated protein with biotin-labeled Datopotamab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Datopotamab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Datopotamab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
78.13 - 5,000 ng/mL
Sensitivity
82.55 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
2635.7
|
603.2
|
131.1
|
2921.6
|
550.4
|
105.1
|
|
Standard deviation
|
432.3
|
110.6
|
25.0
|
427.4
|
107.7
|
21.2
|
|
CV (%)
|
16.4
|
18.3
|
19.0
|
14.6
|
19.6
|
20.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
DS-1062, DS-1062a, Datopotamab deruxtecan, CAS: 2267989-53-5
An evaluation of datopotamab deruxtecan for the treatment of non-small cell lung cancer., PMID:40515573
Efficacy and Safety of TROP-2-Targeting Antibody-Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data., PMID:40507234
Synergistic strategies: ADC-PARP inhibitor combinations in triple-negative breast cancer therapy., PMID:40494034
Perioperative durvalumab plus chemotherapy plus new agents for resectable non-small-cell lung cancer: the platform phase 2 NeoCOAST-2 trial., PMID:40450142
Efficacy and Safety of Antibody-Drug Conjugates for Lung Cancer Therapy: A Systematic Review of Randomized and Non-Randomized Clinical Trials., PMID:40430899
Cost-effectiveness of datopotamab deruxtecan in previously treated advanced nonsquamous NSCLC., PMID:40388791
Decoding Clinical Trials in Metastatic Breast Cancer: Practical Insights for Optimal Therapy Sequencing., PMID:40367401
Datopotamab Deruxtecan: First Approval., PMID:40323341
Preclinical Activity of Datopotamab Deruxtecan, an Antibody-Drug Conjugate Targeting Trophoblast Cell-Surface Antigen 2, in Uterine Serous Carcinoma., PMID:40299780
TROPION-Breast05: a randomized phase III study of Dato-DXd with or without durvalumab versus chemotherapy plus pembrolizumab in patients with PD-L1-high locally recurrent inoperable or metastatic triple-negative breast cancer., PMID:40297626
Therapeutic Potential of Datopotamab Deruxtecan in the Treatment of Advanced Non-Small Cell Lung Cancer: Evidence to Date., PMID:40291609
[Diffuse interstitial lung disease induced by antibody-drug conjugates]., PMID:40263022
Targeting Lung Cancer with Precision: The ADC Therapeutic Revolution., PMID:40238068
FDA approves Datroway: a novel therapy for HR-positive, HER2-negative metastatic breast cancer., PMID:40212175
[Standard of care of EGFR mutated metastatic NSCLC in first treatment and beyond progression]., PMID:40155080
Reply to: Assessing Clinical Utility of Datopotamab Deruxtecan Versus Chemotherapy for Breast Cancer., PMID:40153734
Assessing Clinical Utility of Datopotamab Deruxtecan Versus Chemotherapy for Breast Cancer., PMID:40153615
Prophylaxis, clinical management, and monitoring of datopotamab deruxtecan-associated oral mucositis/stomatitis., PMID:40139260
Antibody-drug conjugates in breast cancer: current evidence and future directions., PMID:40114224
Preclinical evaluation of a novel antibody-drug conjugate OBI-992 for Cancer therapy., PMID:40082588
Antibody-drug conjugates in elderly patients with breast cancer., PMID:40020509
Datopotamab deruxtecan (Datroway) for advanced breast cancer., PMID:40009990
TROPION-Breast04: a randomized phase III study of neoadjuvant datopotamab deruxtecan (Dato-DXd) plus durvalumab followed by adjuvant durvalumab versus standard of care in patients with treatment-naïve early-stage triple negative or HR-low/HER2- breast cancer., PMID:39917260
Beyond failure of endocrine-based therapies in HR+/HER2 negative advanced breast cancer: What before chemotherapy? A glimpse into the future., PMID:39900320
Management of nausea and vomiting induced by antibody-drug conjugates., PMID:39878905
OBI-992, a Novel TROP2-Targeted Antibody-Drug Conjugate, Demonstrates Antitumor Activity in Multiple Cancer Models., PMID:39786401
Datopotamab Deruxtecan in Advanced or Metastatic Non-Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study., PMID:39761483
Antibodies to watch in 2025., PMID:39711140
Efficacy and safety of antibody-drug conjugates in pretreated HER2-low metastatic breast cancer: A systematic review and network meta-analysis., PMID:39709655
Emerging Therapies for Brain Metastases in NSCLC, Breast Cancer, and Melanoma: A Critical Review., PMID:39625633
Antitumor Activity and Biomarker Analysis for TROP2 Antibody-Drug Conjugate Datopotamab Deruxtecan in Patient-Derived Breast Cancer Xenograft Models., PMID:39585341
Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: mechanisms, clinical applications, and future directions., PMID:39555080
TROPION-Lung07: Phase III study of Dato-DXd + pembrolizumab ± platinum-based chemotherapy as 1L therapy for advanced non-small-cell lung cancer., PMID:39469838
Understanding the chemistry & pharmacology of antibody-drug conjugates in triple-negative breast cancer with special reference to exatecan derivatives., PMID:39460856
Can We Find a Place for Trophoblast Cell Surface Antigen 2-Targeted Antibody-Drug Conjugates in Lung Cancer?, PMID:39353165
Datopotamab-deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial., PMID:39277672
Datopotamab-deruxtecan in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial., PMID:39277671
Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: Primary Results From TROPION-Breast01., PMID:39265124
Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non-Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study., PMID:39250535
Preclinical activity of datopotamab deruxtecan, a novel TROP2 directed antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (TROP2) in ovarian carcinoma., PMID:38981151
Blockade of SIRPα-CD47 axis by anti-SIRPα antibody enhances anti-tumor activity of DXd antibody-drug conjugates., PMID:38843278
TROPION-Breast03: a randomized phase III global trial of datopotamab deruxtecan ± durvalumab in patients with triple-negative breast cancer and residual invasive disease at surgical resection after neoadjuvant therapy., PMID:38686016
Perspectives on geriatric oncology research presented at the 2023 San Antonio Breast Cancer Symposium: A Young International Society of Geriatric Oncology report., PMID:38677935
Datopotamab Deruxtecan in Advanced or Metastatic HR+/HER2- and Triple-Negative Breast Cancer: Results From the Phase I TROPION-PanTumor01 Study., PMID:38652877
Antibody-drug conjugates in lung and breast cancer: current evidence and future directions-a position statement from the ETOP IBCSG Partners Foundation., PMID:38648979
Datopotamab deruxtecan: A novel antibody drug conjugate for triple-negative breast cancer., PMID:38560142
Clinical management, monitoring, and prophylaxis of adverse events of special interest associated with datopotamab deruxtecan., PMID:38502995
Best of the year: Advanced breast cancer in 2023., PMID:38401422
Antibodies to watch in 2024., PMID:38178784