Catalog No.
KAV00309
Description
PRINCIPLE OF THE ASSAY
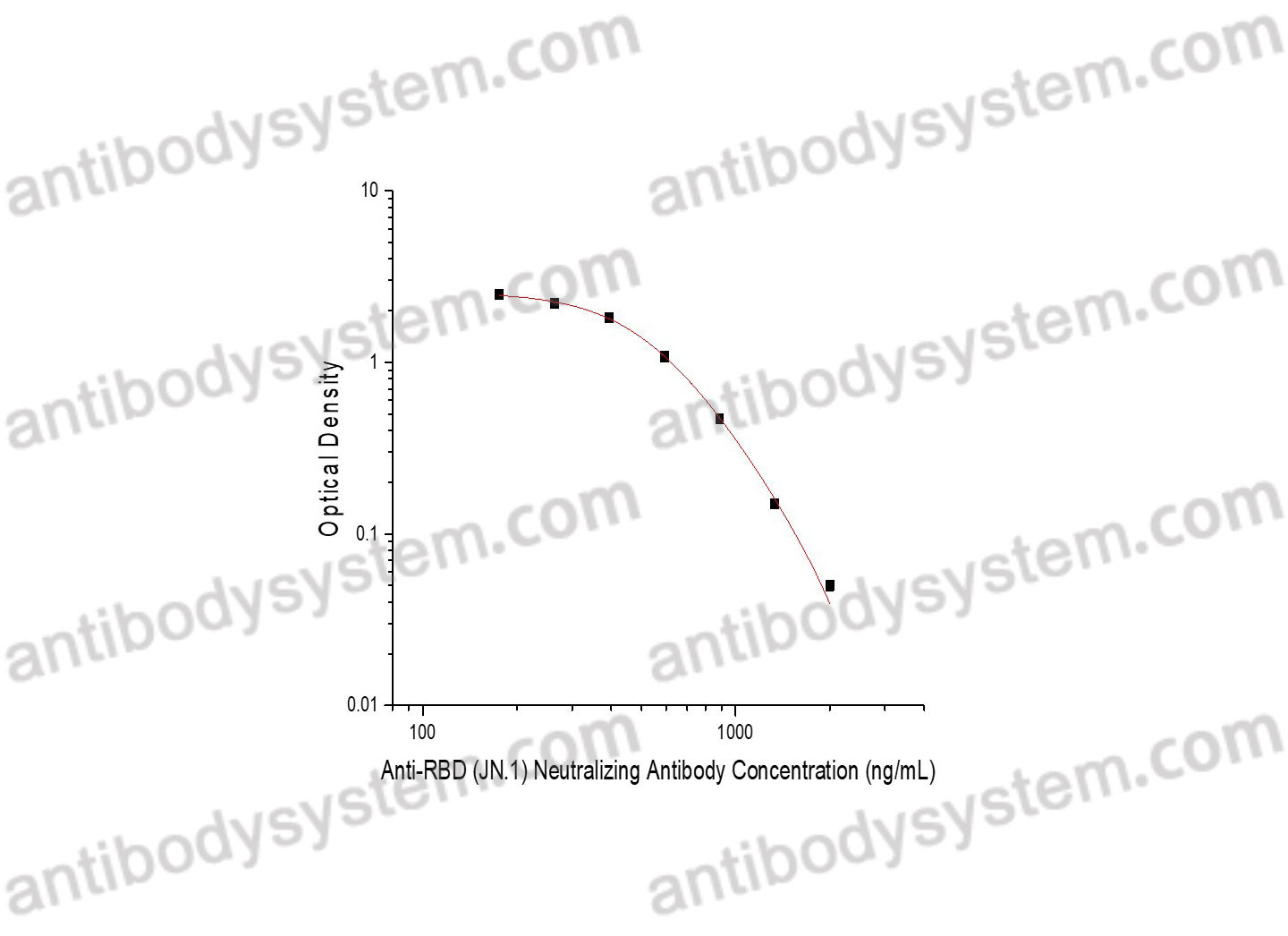
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant RBD (JN.1) has been pre-coated onto a microplate. Standards or samples are premixed with HRP labeled ACE and then pipetted into the wells. Anti-RBD (JN.1) Neutralizing Antibody in the sample competitively binds to recombinant RBD (JN.1) with the HRP labeled ACE. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Anti-RBD (JN.1) Neutralizing Antibody bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
SARS-CoV-2 Surrogate Virus Neutralization Antibody (JN.1)
Applications
Used for the quantitative determination of Anti-RBD (JN.1) Neutralizing Antibody concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
175.58 - 2,000 ng/mL
Sensitivity
175.05 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1797.0
|
958.5
|
497.4
|
1836.6
|
984.7
|
528.7
|
|
Standard deviation
|
58.9
|
71.7
|
29.1
|
68.8
|
63.3
|
58.8
|
|
CV (%)
|
3.3
|
7.5
|
5.9
|
3.7
|
6.4
|
11.1
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Which test results to believe? Comparison of different ELISA kits for detection of SARS-CoV-2-neutralizing antibody among COVID-vaccinated individuals., PMID:40463604
First SARS-CoV-2 Omicron infection as an effective immune booster among mRNA vaccinated individuals: final results from the first phase of the PRIBIVAC randomised clinical trial., PMID:39137572
Anti-SARS-CoV-2 total immunoglobulin and neutralising antibody responses in healthy blood donors throughout the COVID-19 pandemic: a longitudinal observational study., PMID:39137369
Examination of SARS-CoV-2 serological test results from multiple commercial and laboratory platforms with an in-house serum panel., PMID:38482357
Clinical utility of quantitative immunoassays and surrogate virus neutralization tests for predicting neutralizing activity against the SARS-CoV-2 Omicron BA.1 and BA.5 variants., PMID:38140877
Performance of a Point-of-Care Fluorescence Immunoassay Test to Measure the Anti-Severe Acute Respiratory Syndrome Corona Virus 2 Spike, Receptor Binding Domain Antibody Level., PMID:38132270
Impact of Tyrosine Kinase Inhibitors on the Immune Response to SARS-CoV-2 Vaccination in Patients with Non-Small Cell Lung Cancer., PMID:37897014
Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern., PMID:37834413
Performance evaluation of newly developed surrogate virus neutralization tests for detecting neutralizing antibodies against SARS-CoV-2., PMID:36973368
Neutralizing Antibodies as Predictors of Vaccine Breakthrough Infection in Healthcare Workers Vaccinated with or without a Heterologous Booster Dose: A Cohort Study during the Third COVID-19 Wave in Peru., PMID:36851324
Evaluation of a biotin-based surrogate virus neutralization test for detecting postvaccination antibodies against SARS-CoV-2 variants in sera., PMID:36696754
Comprehensive assessment of SARS-CoV-2 antibodies against various antigenic epitopes after naive COVID-19 infection and vaccination (BNT162b2 or ChAdOx1 nCoV-19)., PMID:36578491
Immune Responses against the Omicron Variant of SARS-CoV-2 after a Third Dose of COVID-19 Vaccine in Patients Living with Human Immunodeficiency Virus (PLWH): Comparison with Healthcare Workers., PMID:36560539
Development of in House ELISAs to Detect Antibodies to SARS-CoV-2 in Infected and Vaccinated Humans by Using Recombinant S, S1 and RBD Proteins., PMID:36553092
A highly sensitive bead-based flow cytometric competitive binding assay to detect SARS-CoV-2 neutralizing antibody activity., PMID:36532082
Immunogenicity of COVID-19 vaccines and levels of SARS-CoV-2 neutralising antibody in the Bruneian population: Protocol for a national longitudinal study., PMID:36456015
SARS-CoV-2 neutralizing antibody response after three doses of mRNA1273 vaccine and COVID-19 in hemodialysis patients., PMID:36106337
Neutralizing activity to SARS-CoV-2 in 1.2 to 10.0 month convalescent plasma samples of COVID-19: A transversal surrogate in vitro study performed in Quito-Ecuador., PMID:35585654
Estimating the Neutralizing Effect and Titer Correlation of Semi-Quantitative Anti-SARS-CoV-2 Antibody Immunoassays., PMID:35493733
Efficacy and Safety of Sinopharm Vaccine for SARS-CoV-2 and breakthrough infections in Iranian Patients with Hemoglobinopathies: A Preliminary Report., PMID:35444764
Evaluation of Two Rapid Lateral Flow Tests and Two Surrogate ELISAs for the Detection of SARS-CoV-2 Specific Neutralizing Antibodies., PMID:35187003
Immunogenicity after Second ChAdOx1 nCoV-19 (AZD1222) Vaccination According to the Individual Reactogenicity, Health Status and Lifestyle., PMID:34960219
Two novel SARS-CoV-2 surrogate virus neutralization assays are suitable for assessing successful immunization with mRNA-1273., PMID:34563583
Correlation of sample-to-cut-off ratio of anti-SARS-CoV-2 IgG antibody chemiluminescent assay with neutralization activity: a prospective multi-centric study in India., PMID:34548879
Performance evaluation of three automated quantitative immunoassays and their correlation with a surrogate virus neutralization test in coronavirus disease 19 patients and pre-pandemic controls., PMID:34369009
Serum antibody response to BNT162b2 after natural SARS-CoV-2 infection., PMID:34337738
Evaluation of a surrogate virus neutralization test for high-throughput serosurveillance of SARS-CoV-2., PMID:34224754
Utility of Different Surrogate Enzyme-Linked Immunosorbent Assays (sELISAs) for Detection of SARS-CoV-2 Neutralizing Antibodies., PMID:34069088
Antibody Responses One Year after Mild SARS-CoV-2 Infection., PMID:34060263