Catalog No.
DHD30403
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Human
Isotype
IgG4-kappa
Clonality
Monoclonal
Target
FDC, LAG3, Lymphocyte activation gene 3 protein, CD223, sLAG-3, LAG-3
Concentration
7.5 mg/ml
Endotoxin level
Please contact with the lab for this information.
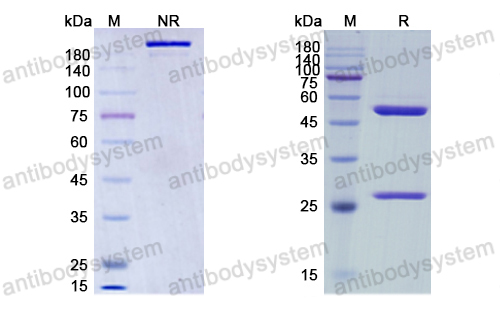
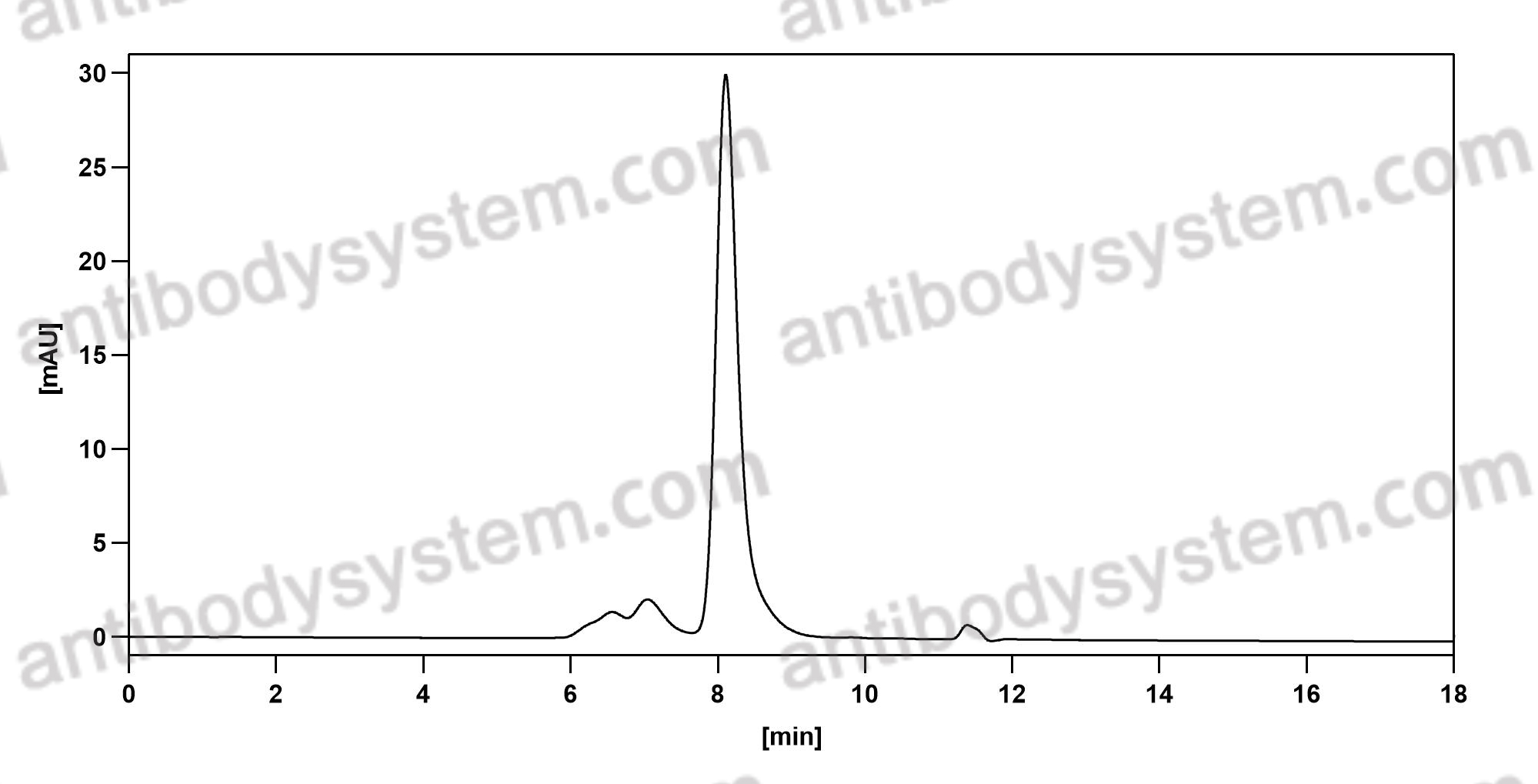
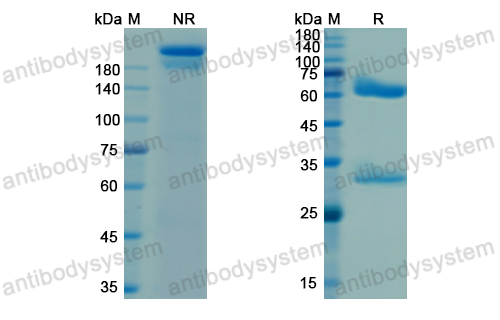
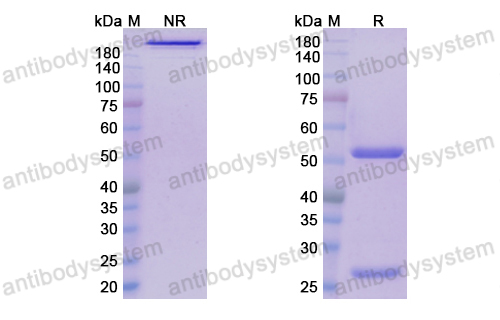
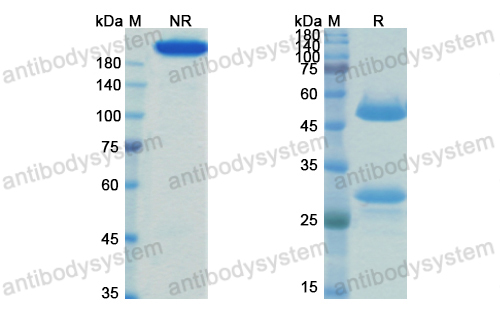
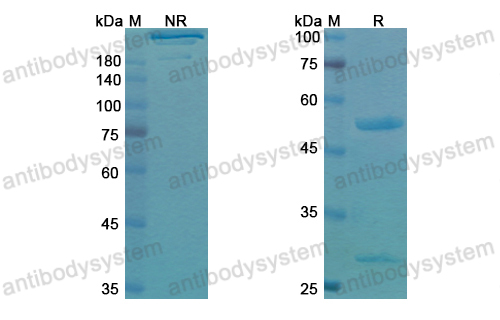
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P18627
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BMS-986016, ONO-4482, CAS: 1673516-98-7
Clone ID
Relatlimab
Characterization of a novel anti-human lymphocyte activation gene 3 (LAG-3) antibody for cancer immunotherapy, PMID: 31242068
LAG-3 Blockade with Relatlimab (BMS-986016) Restores Anti-Leukemic Responses in Chronic Lymphocytic Leukemia, PMID: 33925565
LAG3-PD-1 Combo Impresses in Melanoma, PMID: 34011562
The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients, PMID: 34392069
Exploring new frontiers in LAG-3 biology and therapeutics., PMID:40514283
Nivolumab plus relatlimab in advanced melanoma: RELATIVITY-047 4-year update., PMID:40513285
Immune Checkpoint Inhibitors in the Treatment of Advanced Melanoma in Older Patients: An Overview of Published Data., PMID:40507314
Successful use of nivolumab and relatlimab as salvage therapy for metastatic uveal melanoma., PMID:40465233
Systemic Therapy for Melanoma: What Surgeons Need to Know., PMID:40413004
Cost per outcome of nivolumab + relatlimab vs BRAF + MEK inhibitor combinations for first-line treatment of BRAF-mutant advanced melanoma., PMID:40391872
Rationale and feasibility of a rapid integral biomarker program that informs immune-oncology clinical trials: the ADVISE trial., PMID:40389374
Nivolumab and Relatlimab for the treatment of patients with unresectable or metastatic mismatch repair proficient colorectal cancer., PMID:40388545
An updated systematic review and meta-analysis on the efficacy and safety of nivolumab/relatlimab combination therapy in melanoma patients., PMID:40358773
Complete color vision loss in a patient with metastatic melanoma of the skin treated with nivolumab-relatlimab., PMID:40356467
The emerging role of lymphocyte-activation gene 3 targeting in the treatment of solid malignancies., PMID:40344213
Unlocking LAG3: Ubiquitin's unexpected role., PMID:40315815
Resistance to anti-LAG-3 plus anti-PD-1 therapy in head and neck cancer is mediated by Sox9+ tumor cells interaction with Fpr1+ neutrophils., PMID:40295483
Natural Products for Melanoma Therapy: From Traditional Medicine to Modern Drug Discovery., PMID:40265853
Impact of steroid dose and timing on efficacy of combination PD-1/CTLA-4 blockade., PMID:40248956
Beyond Checkpoint Inhibition: Keeping Therapeutic Options Open., PMID:40233298
Management of metastatic melanoma with combinations including PD-1 inhibitors., PMID:40159098
Immunologic correlates in a CIC::DUX4 fusion-positive sarcoma responsive to dual immune checkpoint blockade., PMID:40128305
Treatment of metastatic melanoma with anti-PD-1 and anti-LAG-3 in a kidney transplant recipient., PMID:40119495
Immune checkpoint Inhibitor related myocarditis reported through the FDA adverse event reporting system: pharmacovigilance trends in reporting and outcomes., PMID:40083879
Erratum: First-Line Nivolumab Plus Relatlimab Versus Nivolumab Plus Ipilimumab in Advanced Melanoma: An Indirect Treatment Comparison Using RELATIVITY-047 and CheckMate 067 Trial Data., PMID:40053890
Nivolumab plus relatlimab in patients with relapsed or progressed B-cell malignancies in RELATIVITY-022., PMID:40030000
Nivolumab/relatlimab-associated cutaneous immune-related adverse events in patients treated for advanced melanoma: A multicenter retrospective cohort study., PMID:40024392
Effect of prior and first-line immunotherapy on baseline immune biomarkers and modulation of the tumor microenvironment in response to nivolumab and relatlimab combination therapy in patients with melanoma from RELATIVITY-020., PMID:40010775
The Risk of Adrenal Insufficiency after Treatment with Relatlimab in Combination with Nivolumab is Higher than Expected., PMID:40001294
Tri-specific tribodies targeting 5T4, CD3, and immune checkpoint drive stronger functional T-cell responses than combinations of antibody therapeutics., PMID:39929828
Challenges and prospects of LAG-3 inhibition in advanced gastric and gastroesophageal junction cancer: insights from the RELATIVITY-060 trial., PMID:39816014
Nivolumab combination therapies in patients with advanced gastric and gastroesophageal junction cancer: the phase II FRACTION gastric cancer study., PMID:39798422
An update on access to novel treatment for metastatic melanoma in Europe - A 2024 survey of the European melanoma registry and the European association of dermato-oncology., PMID:39721295
Three-Year Overall Survival With Nivolumab Plus Relatlimab in Advanced Melanoma From RELATIVITY-047., PMID:39671533
Nivolumab plus relatlimab and nivolumab plus ipilimumab for patients with advanced renal cell carcinoma: results from the open-label, randomised, phase II FRACTION-RCC trial., PMID:39642635
The efficacy and safety of relatlimab/nivolumab combination therapy in patients with advanced melanoma: a systematic review., PMID:39636334
Real world comparison of immune-related adverse events with nivolumab-relatlimab versus ipilimumab-nivolumab in patients with advanced cutaneous melanoma., PMID:39635988
Immune Checkpoint Inhibitor Myopathy: The Double-Edged Sword of Cancer Immunotherapy., PMID:39514829
A comprehensive review of immune checkpoint inhibitors for cancer treatment., PMID:39447408
Deciphering LAG-3: unveiling molecular mechanisms and clinical advancements., PMID:39425148
Case report: Conjunctival melanoma treated with relatlimab and nivolumab showing remarkable response., PMID:39386188
Real-world treatment patterns and outcomes of patients with advanced melanoma treated with nivolumab plus relatlimab., PMID:39293068
Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial., PMID:39278994
Advances in understanding the role of immune checkpoint LAG-3 in tumor immunity: a comprehensive review., PMID:39252941
Updates and emerging trends in the management of immune-related adverse events associated with immune checkpoint inhibitor therapy., PMID:39234578
First-Line Nivolumab Plus Relatlimab Versus Nivolumab Plus Ipilimumab in Advanced Melanoma: An Indirect Treatment Comparison Using RELATIVITY-047 and CheckMate 067 Trial Data., PMID:39137386
Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8+ T cells to promote antitumor immunity., PMID:39121849
LAG-3 and PD-1 synergize on CD8+ T cells to drive T cell exhaustion and hinder autocrine IFN-γ-dependent anti-tumor immunity., PMID:39121848
Adverse Events of PD-1, PD-L1, CTLA-4, and LAG-3 Immune Checkpoint Inhibitors: An Analysis of the FDA Adverse Events Database., PMID:39051335
Nivolumab Reaches Brain Lesions in Patients with Recurrent Glioblastoma and Induces T-cell Activity and Upregulation of Checkpoint Pathways., PMID:38885356
Nivolumab plus relatlimab in patients with previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study., PMID:38821718
A novel LAG3 neutralizing antibody improves cancer immunotherapy by dual inhibition of MHC-II and FGL1 ligand binding., PMID:38776682
Project Optimus Elicits the "Holistic" Benefits of PK/PD Modeling of Immunotherapy., PMID:38743418
Immune Checkpoint Inhibitor Induced Supraglottitis: A Case Series., PMID:38742617