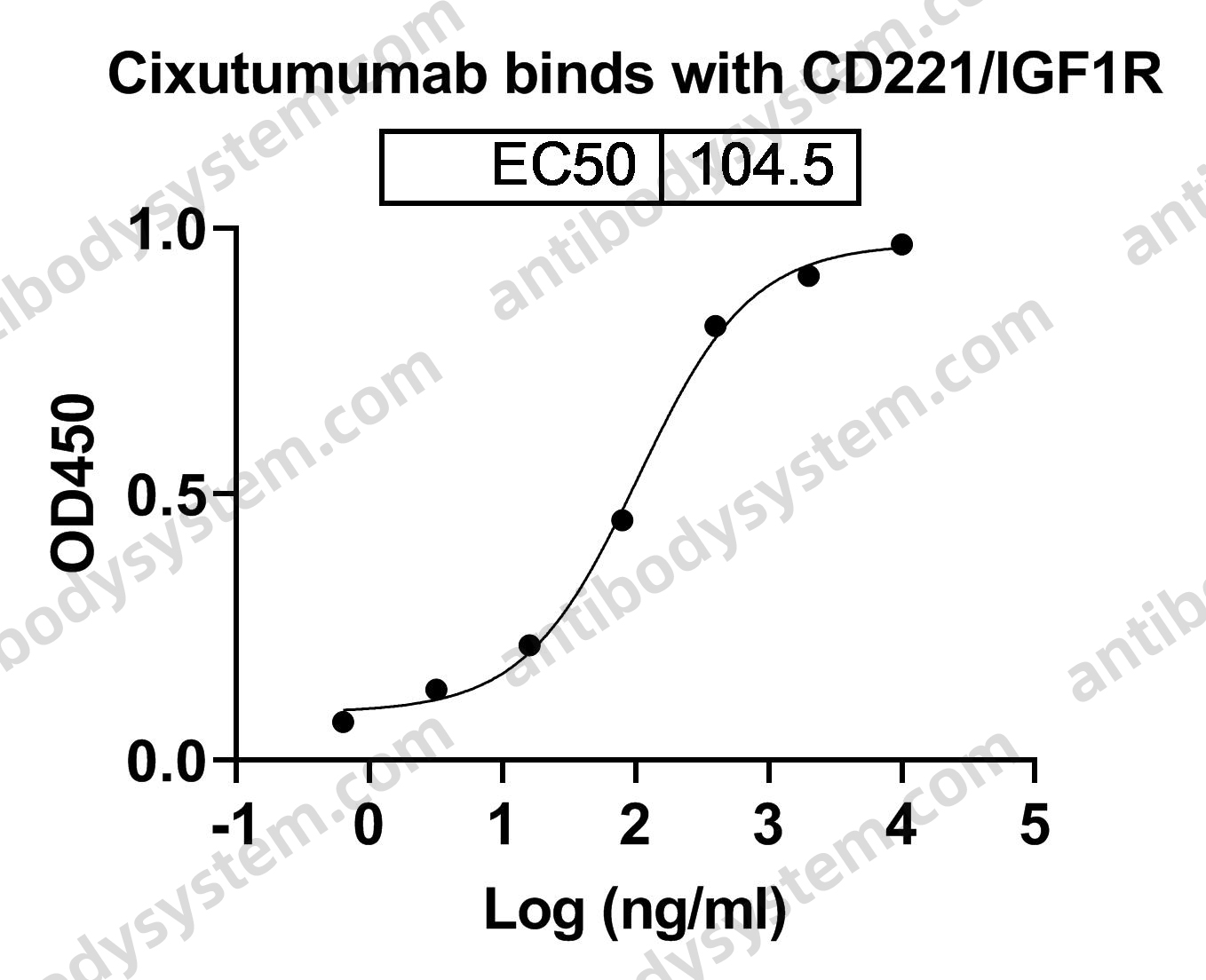
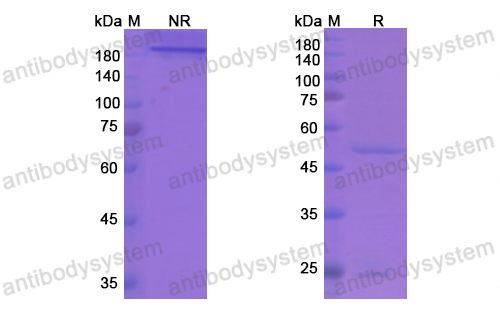
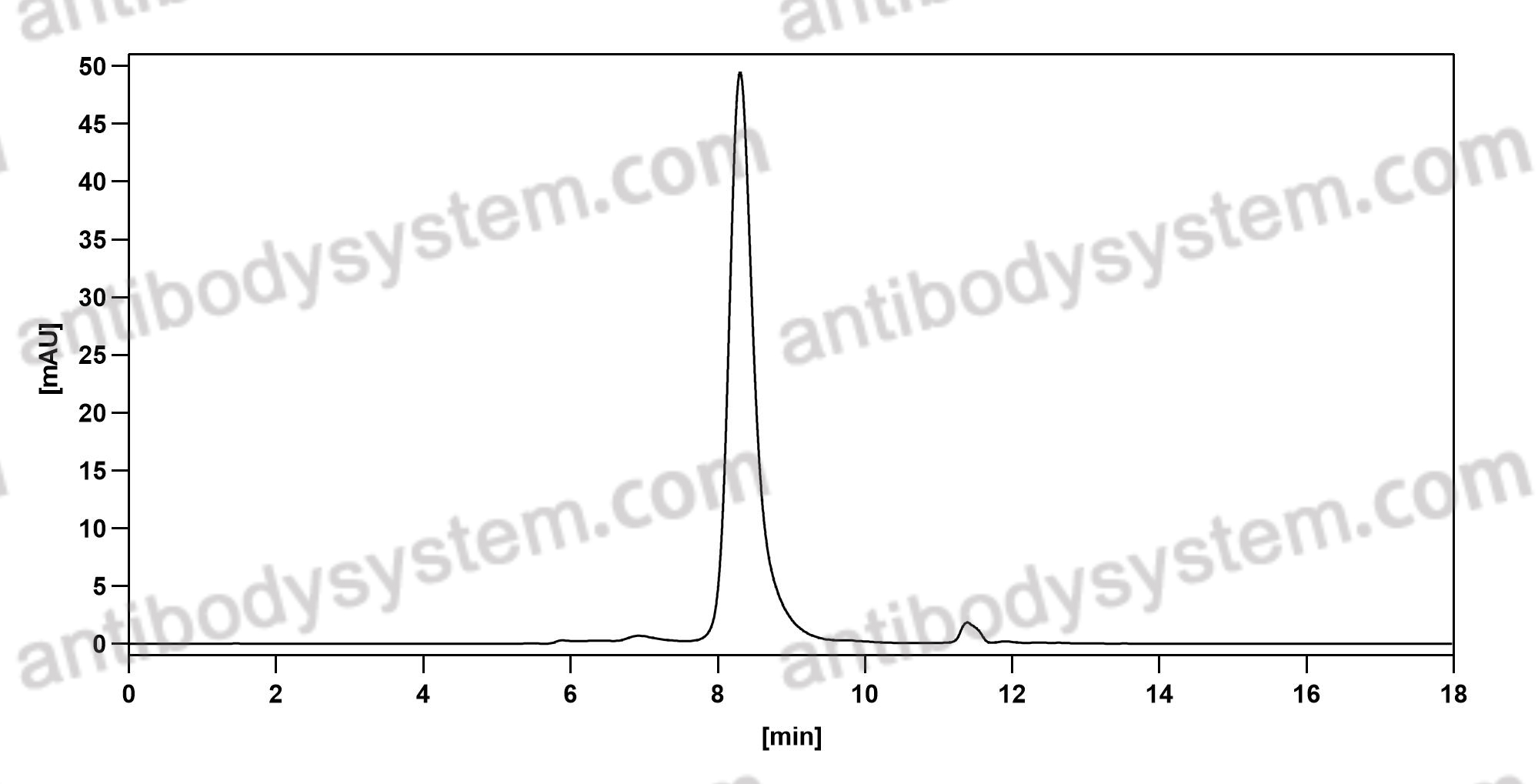
Cixutumumab, PMID: 19548856
Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer, PMID: 33122707
Adverse Events and Efficacy of Cixutumumab in Phase II Clinical Trials: A systematic Review and Meta-Analysis, PMID: 27858328
111 In- and 225 Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer, PMID: 31518138
Blockade of insulin-like growth factor type-1 receptor with cixutumumab (IMC-A12): a novel approach to treatment for multiple cancers, PMID: 21777192
The addition of cixutumumab or temozolomide to intensive multiagent chemotherapy is feasible but does not improve outcome for patients with metastatic rhabdomyosarcoma: A report from the Children's Oncology Group, PMID: 30351457
Intrinsic Resistance to Cixutumumab Is Conferred by Distinct Isoforms of the Insulin Receptor, PMID: 26263910
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy, PMID: 28427155
A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma, PMID: 29520435
Survival outcomes and risk group validation from SWOG S0925: a randomized phase II study of cixutumumab in new metastatic hormone-sensitive prostate cancer, PMID: 32055002
Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial, PMID: 24439931
A Phase I Trial of IGF-1R Inhibitor Cixutumumab and mTOR Inhibitor Temsirolimus in Metastatic Castration-resistant Prostate Cancer, PMID: 32057715
Cixutumumab and temsirolimus for patients with bone and soft-tissue sarcoma: a multicentre, open-label, phase 2 trial, PMID: 23477833
Phase I trial of cixutumumab combined with temsirolimus in patients with advanced cancer, PMID: 21750201
Randomized phase II trial of cixutumumab alone or with cetuximab for refractory recurrent/metastatic head and neck squamous cell carcinoma, PMID: 29909907
Phase 2 trial of cixutumumab in children, adolescents, and young adults with refractory solid tumors: a report from the Children's Oncology Group, PMID: 23956055
Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors, PMID: 25749986
An Open-Label, Multicenter, Randomized, Phase II Study of Cisplatin and Pemetrexed With or Without Cixutumumab (IMC-A12) as a First-Line Therapy in Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer, PMID: 27464970
Efficacy of anti-insulin-like growth factor I receptor monoclonal antibody cixutumumab in mesothelioma is highly correlated with insulin growth factor-I receptor sites/cell, PMID: 22323052
A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer, PMID: 26082390
SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer, PMID: 25847934
Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children's Oncology Group, PMID: 22184397
Insulin-like growth factor-I receptor (IGF-IR) targeting with monoclonal antibody cixutumumab (IMC-A12) inhibits IGF-I action in endometrial cancer cells, PMID: 21450456
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model, PMID: 25858498
Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727), PMID: 25041791
Clinical and Translational Results of a Phase II, Randomized Trial of an Anti-IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy, PMID: 26324738
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model, PMID: 30726873
A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma, PMID: 24045151
An open-label, phase 2 study evaluating the efficacy and safety of the anti-IGF-1R antibody cixutumumab in patients with previously treated advanced or metastatic soft-tissue sarcoma or Ewing family of tumours, PMID: 23835252
Phase II randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: a trial of the ECOG-ACRIN Cancer Research Group (E3508), PMID: 28950351
Combating resistance to anti-IGFR antibody by targeting the integrin β3-Src pathway, PMID: 24092920
Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors, PMID: 25900182
A Phase I Study of Cixutumumab (IMC-A12) in Combination with Temsirolimus (CCI-779) in Children with Recurrent Solid Tumors: A Children's Oncology Group Phase I Consortium Report, PMID: 25467181
Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508), PMID: 27163943
The Prevalence and Impact of Hyperglycemia and Hyperlipidemia in Patients With Advanced Cancer Receiving Combination Treatment With the Mammalian Target of Rapamycin Inhibitor Temsirolimus and Insulin Growth Factor-Receptor Antibody Cixutumumab, PMID: 26054632
The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial, PMID: 24849545
A phase I trial of the IGF-1R antibody Cixutumumab in combination with temsirolimus in patients with metastatic breast cancer, PMID: 23605083
A phase I study evaluating cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, given every 2 or 3 weeks in Japanese patients with advanced solid tumors, PMID: 27139036
A phase I/II study of erlotinib in combination with the anti-insulin-like growth factor-1 receptor monoclonal antibody IMC-A12 (cixutumumab) in patients with advanced non-small cell lung cancer, PMID: 22237261
A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours, PMID: 25268371
Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma, PMID: 23412108
Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group, PMID: 25446280
Akt/mTOR counteract the antitumor activities of cixutumumab, an anti-insulin-like growth factor I receptor monoclonal antibody, PMID: 21980128
Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing's sarcoma family tumors, PMID: 22465830
Three-arm, randomized, phase 2 study of carboplatin and paclitaxel in combination with cetuximab, cixutumumab, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: An Eastern Cooperative Oncology Group (ECOG) study (E4508), PMID: 25740387
A meta-analysis of CXCL12 expression for cancer prognosis, PMID: 28535157
Novel agents in early phase clinical studies on refractory pancreatic cancer, PMID: 22406592
Case series of dermatologic events associated with the insulin-like growth factor receptor 1 inhibitor cixutumumab, PMID: 21606420
Electrolyte disorders associated with the use of anticancer drugs, PMID: 26939882
Cixutumumab-associated pancolitis in a patient with prostate cancer, PMID: 23083799
Paclitaxel With or Without Cixutumumab as Second-Line Treatment of Metastatic Esophageal or Gastroesophageal Junction Cancer: A Randomized Phase II ECOG-ACRIN Trial., PMID:37104870
Comparison of Second-Line Treatments for Patients with Platinum-Resistant Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Systematic Review and Bayesian Network Meta-Analysis., PMID:36139631
Cixutumumab reveals a critical role for IGF-1 in adipose and hepatic tissue remodelling during the development of diet-induced obesity., PMID:35734881
The Immunotherapy Landscape in Adrenocortical Cancer., PMID:34071333
Randomized Phase II Trial of Capecitabine and Lapatinib with or without IMC-A12 (Cituxumumab) in Patients with HER2-Positive Advanced Breast Cancer Previously Treated with Trastuzumab and Chemotherapy: NCCTG N0733 (Alliance)., PMID:33852121
An expanded genetic code facilitates antibody chemical conjugation involving the lambda light chain., PMID:33561746
Development and preclinical evaluation of cixutumumab drug conjugates in a model of insulin growth factor receptor I (IGF-1R) positive cancer., PMID:33122707
The Receptor Tyrosine Kinase RON and Its Isoforms as Therapeutic Targets in Ewing Sarcoma., PMID:32272784
A Phase I Trial of IGF-1R Inhibitor Cixutumumab and mTOR Inhibitor Temsirolimus in Metastatic Castration-resistant Prostate Cancer., PMID:32057715
Survival outcomes and risk group validation from SWOG S0925: a randomized phase II study of cixutumumab in new metastatic hormone-sensitive prostate cancer., PMID:32055002
111In- and 225Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer., PMID:31518138
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model., PMID:30726873
The addition of cixutumumab or temozolomide to intensive multiagent chemotherapy is feasible but does not improve outcome for patients with metastatic rhabdomyosarcoma: A report from the Children's Oncology Group., PMID:30351457
Randomized phase II trial of cixutumumab alone or with cetuximab for refractory recurrent/metastatic head and neck squamous cell carcinoma., PMID:29909907
A phase I trial of escalating doses of cixutumumab (IMC-A12) and sorafenib in the treatment of advanced hepatocellular carcinoma., PMID:29520435
Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer., PMID:29105802
Phase II randomized trial of carboplatin, paclitaxel, bevacizumab with or without cixutumumab (IMC-A12) in patients with advanced non-squamous, non-small-cell lung cancer: a trial of the ECOG-ACRIN Cancer Research Group (E3508)., PMID:28950351
A meta-analysis of CXCL12 expression for cancer prognosis., PMID:28535157
Update of IGF-1 receptor inhibitor (ganitumab, dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on cancer therapy., PMID:28427155
In silico prediction of B cell epitopes of the extracellular domain of insulin-like growth factor-1 receptor., PMID:28261624
Adverse Events and Efficacy of Cixutumumab in Phase II Clinical Trials: A systematic Review and Meta-Analysis., PMID:27858328
Mortality Among Men with Advanced Prostate Cancer Excluded from the ProtecT Trial., PMID:27720537
An Open-Label, Multicenter, Randomized, Phase II Study of Cisplatin and Pemetrexed With or Without Cixutumumab (IMC-A12) as a First-Line Therapy in Patients With Advanced Nonsquamous Non-Small Cell Lung Cancer., PMID:27464970
The TGFβ pathway stimulates ovarian cancer cell proliferation by increasing IGF1R levels., PMID:27299695
Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508)., PMID:27163943
A phase I study evaluating cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, given every 2 or 3 weeks in Japanese patients with advanced solid tumors., PMID:27139036
Electrolyte disorders associated with the use of anticancer drugs., PMID:26939882
STAT3-mediated IGF-2 secretion in the tumour microenvironment elicits innate resistance to anti-IGF-1R antibody., PMID:26465273
Molecular Pathways: Clinical Applications and Future Direction of Insulin-like Growth Factor-1 Receptor Pathway Blockade., PMID:26429980
Clinical and Translational Results of a Phase II, Randomized Trial of an Anti-IGF-1R (Cixutumumab) in Women with Breast Cancer That Progressed on Endocrine Therapy., PMID:26324738
Intrinsic Resistance to Cixutumumab Is Conferred by Distinct Isoforms of the Insulin Receptor., PMID:26263910
A randomised non-comparative phase II trial of cixutumumab (IMC-A12) or ramucirumab (IMC-1121B) plus mitoxantrone and prednisone in men with metastatic docetaxel-pretreated castration-resistant prostate cancer., PMID:26082390
The Prevalence and Impact of Hyperglycemia and Hyperlipidemia in Patients With Advanced Cancer Receiving Combination Treatment With the Mammalian Target of Rapamycin Inhibitor Temsirolimus and Insulin Growth Factor-Receptor Antibody Cixutumumab., PMID:26054632
Clinical efficacy of mTOR inhibitors in solid tumors: a systematic review., PMID:26043220
Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors., PMID:25900182
Doxorubicin plus the IGF-1R antibody cixutumumab in soft tissue sarcoma: a phase I study using the TITE-CRM model., PMID:25858498
SWOG S0925: A Randomized Phase II Study of Androgen Deprivation Combined With Cixutumumab Versus Androgen Deprivation Alone in Patients With New Metastatic Hormone-Sensitive Prostate Cancer., PMID:25847934
Targeted therapy for thymic epithelial tumors: a new horizon? Review of the literature and two cases reports., PMID:25832879
Do thymic malignancies respond to target therapies?, PMID:25754373
Safety, tolerability, and pharmacokinetics of single and multiple doses of intravenous cixutumumab (IMC-A12), an inhibitor of the insulin-like growth factor-I receptor, administered weekly or every 2 weeks in patients with advanced solid tumors., PMID:25749986
Three-arm, randomized, phase 2 study of carboplatin and paclitaxel in combination with cetuximab, cixutumumab, or both for advanced non-small cell lung cancer (NSCLC) patients who will not receive bevacizumab-based therapy: An Eastern Cooperative Oncology Group (ECOG) study (E4508)., PMID:25740387
Inhibition of intracerebral glioblastoma growth by targeting the insulin-like growth factor 1 receptor involves different context-dependent mechanisms., PMID:25543125
A Phase I Study of Cixutumumab (IMC-A12) in Combination with Temsirolimus (CCI-779) in Children with Recurrent Solid Tumors: A Children's Oncology Group Phase I Consortium Report., PMID:25467181
Phase II study of cixutumumab in combination with temsirolimus in pediatric patients and young adults with recurrent or refractory sarcoma: a report from the Children's Oncology Group., PMID:25446280
A phase I trial of vertical inhibition of IGF signalling using cixutumumab, an anti-IGF-1R antibody, and selumetinib, an MEK 1/2 inhibitor, in advanced solid tumours., PMID:25268371
Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727)., PMID:25041791
The combination of insulin-like growth factor receptor 1 (IGF1R) antibody cixutumumab and mitotane as a first-line therapy for patients with recurrent/metastatic adrenocortical carcinoma: a multi-institutional NCI-sponsored trial., PMID:24849545
Novel biologic therapies for thymic epithelial tumors., PMID:24847446
Incidence of mucositis in patients treated with temsirolimus-based regimens and correlation to treatment response., PMID:24668327
Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance--implications for IGF-II and IGF-IR-targeted therapy., PMID:24599933