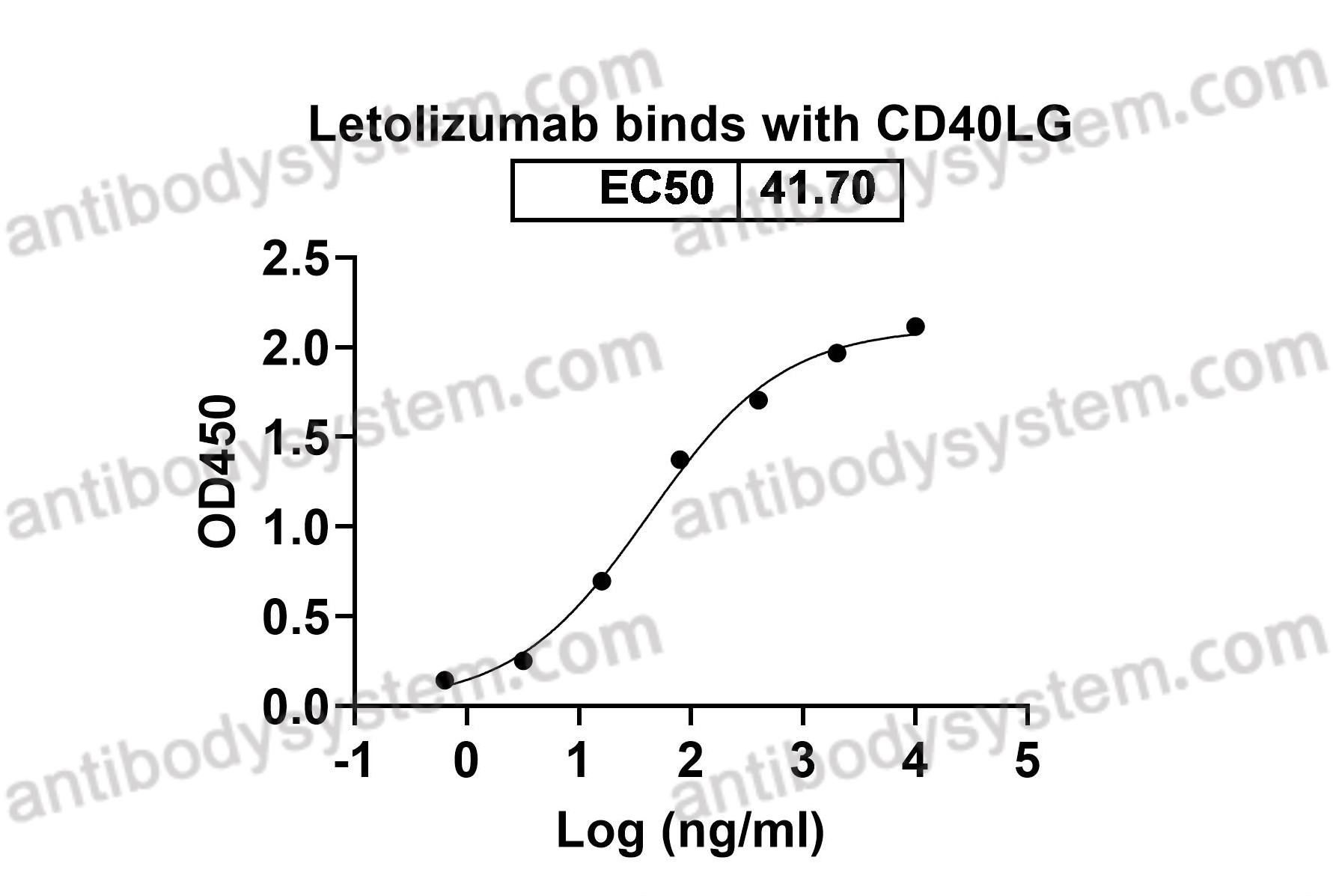
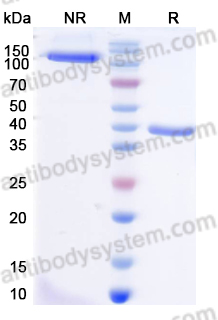
Catalog No.
DHD85401
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
(VH-CH2-CH3)2
Clonality
Monoclonal
Target
sCD40L, Tumor necrosis factor ligand superfamily member 5, T-cell antigen Gp39, CD40L, TNFSF5, CD154, CD40-L, TNF-related activation protein, CD40 ligand, CD40LG, TRAP
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P29965
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
BMS-986004, CAS: 1450981-87-9
Clone ID
Letolizumab
Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications, PMID: 32700604
Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis, PMID: 33378357
Possible treatment and strategies for COVID-19: review and assessment, PMID: 33336780
Itolizumab in Psoriasis, PMID: 28794555
Spotlight on itolizumab in the treatment of psoriasis - current perspectives from India, PMID: 31119094
Antibodies to watch in 2021, PMID: 33459118
Itolizumab, a novel anti-CD6 monoclonal antibody: a safe and efficacious biologic agent for management of psoriasis, PMID: 28064543
Approval of Itolizumab for COVID-19: A Premature Decision or Need of The Hour?, PMID: 33048300
Treatment of COVID-19 patients with the anti-CD6 antibody itolizumab, PMID: 33304584
Are We Jumping the Gun with Itolizumab in India? A Situational Analysis from the Pre-COVID Era, PMID: 33015549
Itolizumab - a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis, PMID: 25945063
Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review, PMID: 33068263
Use of a Humanized Anti-CD6 Monoclonal Antibody (Itolizumab) in Elderly Patients with Moderate COVID-19, PMID: 33105142
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis, PMID: 29271481
Therapeutic Targeting of CD6 in Autoimmune Diseases: A Review of Cuban Clinical Studies with the Antibodies IOR-T1 and Itolizumab, PMID: 26844560
New Targeted Therapies for Uncontrolled Asthma, PMID: 31076057
An anti-CD6 monoclonal antibody (itolizumab) reduces circulating IL-6 in severe COVID-19 elderly patients, PMID: 33292350
Itolizumab in the Management of Psoriasis with Metabolic Syndrome, PMID: 28893023
Itolizumab in combination with methotrexate modulates active rheumatoid arthritis: safety and efficacy from a phase 2, randomized, open-label, parallel-group, dose-ranging study, PMID: 26050104
Itolizumab - A New Biologic for Management of Psoriasis and Psoriatic Arthritis, PMID: 29033818
Itolizumab Treatment for Cytokine Release Syndrome in Moderate to Severe Acute Respiratory Distress Syndrome Due to COVID-19: Clinical Outcomes, A Retrospective Study, PMID: 33527804
The anti-CD6 antibody itolizumab provides clinical benefit without lymphopenia in rheumatoid arthritis patients: results from a 6-month, open-label Phase I clinical trial, PMID: 28963724
Immunological evaluation of rheumatoid arthritis patients treated with itolizumab, PMID: 26466969
A Real-World Study to Assess the Effectiveness of Itolizumab in Patients with Chronic Plaque Psoriasis, PMID: 28761839
Itolizumab provides sustained remission in plaque psoriasis: a 5-year follow-up experience, PMID: 25495868
T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab, PMID: 28672038
Clinical Outcome of a Novel Anti-CD6 Biologic Itolizumab in Patients of Psoriasis with Comorbid Conditions, PMID: 27885324
Off-label Use of Itolizumab in Patients with COVID-19 ARDS: Our Clinical Experience in a Dedicated COVID Center, PMID: 34045817
Safety and Efficacy of Itolizumab in the Treatment of Psoriasis: A Case Series of 20 Patients, PMID: 28050487
Tailored treatment options for patients with psoriatic arthritis and psoriasis: review of established and new biologic and small molecule therapies, PMID: 26892034
Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study, PMID: 24703722
Rationale for treating primary Sjögren's syndrome patients with an anti-CD6 monoclonal antibody (Itolizumab), PMID: 23576060
A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis, PMID: 24371585
Severe recalcitrant psoriasis treated with itolizumab, a novel anti-CD6 monoclonal antibody, PMID: 27279321
Correction: T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab, PMID: 29381749
Erratum: Itolizumab - a humanized anti-CD6 monoclonal antibody with better side effects profile for the treatment of psoriasis [Corrigendum], PMID: 26082656
Anti-CD6 mAbs for the treatment of psoriasis, PMID: 32466689
Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab, PMID: 24594862
Potential effects of itolizumab treatment on plasma interleukin-6 levels in patients with severe COVID-19, PMID: 34169913
Second-degree Heart Block Caused by Itolizumab-induced Infusion Reaction in COVID-19, PMID: 34045820
Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled study, PMID: 26183983
A two-arm, randomized, controlled, multi-centric, open-label phase-2 study to evaluate the efficacy and safety of Itolizumab in moderate to severe ARDS patients due to COVID-19, PMID: 33835886
Scientists criticize use of unproven COVID drugs in India, PMID: 33169025
Clinical and experimental evidence for targeting CD6 in immune-based disorders, PMID: 29526637
Antibodies to watch in 2013: Mid-year update, PMID: 23727858
CD6 monoclonal antibodies differ in epitope, kinetics and mechanism of action, PMID: 29772075
Biologics use in Indian psoriasis patients, PMID: 27990383
Which are the antibodies to watch in 2013?, PMID: 23254906