Catalog No.
KAK13901
Description
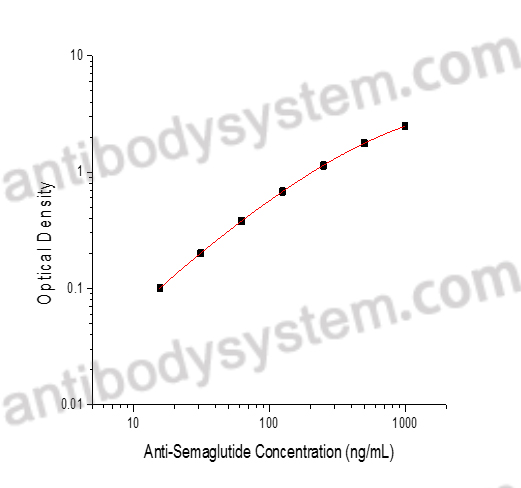
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Semaglutide has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-Semaglutide present is bound by the immobilized protein. After washing away any unbound substances, a HRP conjugated probe is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-Semaglutide bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Anti-Semaglutide concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
15.63 - 1,000 ng/mL
Sensitivity
6.49 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
519.9
|
135.2
|
30.9
|
542.8
|
112.8
|
17.3
|
|
Standard deviation
|
28.3
|
9.5
|
2.0
|
71.5
|
16.7
|
2.5
|
|
CV (%)
|
5.5
|
7.1
|
6.4
|
13.2
|
14.8
|
14.7
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%. For unopened kits, if you want to prolong the storage time, please store the Standard, Detection A, Detection B and Microplate at - 20 ℃, the rest reagents should be store at 4℃.
Alternative Names
CAS, 910463-68-2, Semaglutide, Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217
Progress and challenges in obesity pharmacotherapy: semaglutide as a milestone., PMID:40515833
[Weight reduction with incretin mimetics-Opportunities and risks]., PMID:40514554
A biodegradable suction patch for sustainable transbuccal peptide delivery., PMID:40513668
Controversies in nomenclature: From nonalcoholic steatohepatitis to the full spectrum of hepatic steatosis., PMID:40513177
2025 ACC Scientific Statement on the Management of Obesity in Adults With Heart Failure: A Report of the American College of Cardiology., PMID:40512113
Addressing obesity and subsequent cardiovascular risk in primary care: the relevance of high- and low-resource settings., PMID:40512090
Key takeaways from the updated multidisciplinary European MASLD guidelines., PMID:40510733
Utilization Trends of Dual GIP/GLP-1 Receptor Agonist, Newer Glucose-Lowering Medications, and Anti-Obesity Medications Among Patients With Chronic Kidney Disease With and Without Type 2 Diabetes., PMID:40510603
Oral semaglutide for the treatment of obesity: a retrospective real-world study., PMID:40510489
Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies., PMID:40507553
Efficacy and safety of semaglutide versus placebo for people with schizophrenia on clozapine with obesity (COaST): a phase 2, multi-centre, participant and investigator- blinded, randomised controlled trial in Australia., PMID:40506208
Semaglutide for clozapine-treated patients with schizophrenia., PMID:40506202
Comparative Efficacy of Tirzepatide vs. Semaglutide in Reducing Body Weight in Humans: A Systematic Review and Meta-Analysis of Clinical Trials and Real-World Data., PMID:40503067
Efficacy of the GLP-1 receptor agonist, semaglutide, in abstinence from illicit and nonprescribed opioids in an outpatient population with treatment-refractory OUD: A randomized, double-blind, placebo-controlled clinical trial protocol., PMID:40502777
Real-world comparative outcomes of GLP-1 RA and semaglutide prescription among individuals with type 2 diabetes., PMID:40502555
Effect of Semaglutide on C-peptide levels in patients with type 2 diabetes., PMID:40501244
Semaglutide-A ray of hope for CKD in diabetic patients., PMID:40500852
Design, synthesis, and biological evaluation of long-acting glucagon-like peptide-1 (GLP-1) conjugates modified with dual fatty acids and a proline-alanine-serine (PAS) polypeptide., PMID:40499315
The Impact of New Weight-Loss Medications on Bariatric Surgery and Surgeon Employment., PMID:40497223
Clinical Profile and Prognosis of Patients With Acute Decompensated Heart Failure Who Met the Obesity-Related Eligibility for Subcutaneous Semaglutide - Findings From the CURE-HF Registry., PMID:40497126
Mitochondrial dysfunction characterises the multigenerational effects of maternal obesity on MASLD., PMID:40496439
Finerenone and semaglutide: Role in heart failure with reduced ejection fraction., PMID:40496391
Neuroprotective and cognitive benefits of Semaglutide: Insights into the underlying molecular mechanisms., PMID:40494410
Meta-analysis of the Effect of Semaglutide on Blood Pressure in Obese Populations., PMID:40493329
Impact of semaglutide on health outcomes and mood in obese heart failure patients: a retrospective analysis., PMID:40493069
Changes in weight and glycemic control following obesity treatment with semaglutide or tirzepatide by discontinuation status., PMID:40491239
Harnessing Surface Hydrophilicity of Inhalable Nanoparticles for Precision Delivery of Glucagon-like Peptide-1 Receptor Agonists or Anti-Asthmatic Therapeutics., PMID:40490304
Drug-related visual blurring: findings from the U.S. Food and Drug Administration Adverse Event Reporting System database., PMID:40490170
Effect of pH, buffers, molarity, and temperature on solution state degradation of semaglutide using LC-HRMS: A preformulation protocol for peptide drug delivery., PMID:40490042
Efficacy of GLP-1-based Therapies on Metabolic Dysfunction-Associated Steatotic Liver Disease and Metabolic Dysfunction-Associated Steatohepatitis: A Systematic Review and Meta-Analysis., PMID:40489581
External control arm with real world data to assess the effect of semaglutide on chronic kidney disease risk among patients with type 2 diabetes., PMID:40489337
Worth the Weight? The Challenges of Administering the Glucagon-Like Peptide 1 Receptor Agonist Semaglutide With Long-Term Olanzapine Use in a Patient With Schizophrenia., PMID:40488719
Association of GLP-1 Receptor Agonists with Liver-Related Outcomes and All-Cause Mortality in Patients with Harmful Alcohol Use: A Target Trial Emulation Study., PMID:40488647
Antiemetic effect of acupressure wristbands for GLP-1 medication associated nausea., PMID:40487675
Pregnancy outcomes following first trimester exposure to semaglutide., PMID:40487375
Improved total shoulder arthroplasty outcomes associated with semaglutide utilization in patients with type II diabetes: a promising new addition to preoperative optimization., PMID:40486798
Effects of Tirzepatide on Patients With Type 2 Diabetes and Metabolic Dysfunction-Associated Steatotic Liver Disease: A Retrospective Cohort Study., PMID:40486301
GLP-1 receptor agonists are associated with fewer major adverse cardiovascular and limb events in patients with moderate PAD., PMID:40484062
Once‑weekly IcoSema versus once‑weekly insulin icodec in type 2 diabetes management (COMBINE 1): an open‑label, multicentre, treat‑to‑target, randomised, phase 3a trial., PMID:40482671
Once-weekly IcoSema versus multiple daily insulin injections in type 2 diabetes management (COMBINE 3): an open-label, multicentre, treat-to-target, non-inferiority, randomised, phase 3a trial., PMID:40482670
The Obesity Paradox of Cardiovascular Outcomes in Patients with Diabetes Mellitus., PMID:40481922
Tirzepatide Outperforms Semaglutide in Head-to-Head Obesity Trial., PMID:40478570
Research letter: the global clinical trial landscape for non-alcoholic fatty liver disease (NAFLD) current status and future prospects., PMID:40474833
Efficacy of Semaglutide Injection in the Treatment of Type 2 Diabetes Mellitus and its Impact on C-Peptide Levels., PMID:40473254
CROI 2025: neuropsychiatric complications in people with HIV., PMID:40472384
Prescriptions for Obesity Medications Among Adolescents Aged 12-17 Years with Obesity - United States, 2018-2023., PMID:40471858
Semaglutide Modulates Proinflammatory Epicardial Adipogenesis With Paracrine Effects on hiPSC-Atrial Cardiomyocytes., PMID:40471762
Beyond GLP-1: efficacy and safety of dual and triple incretin agonists in personalized type 2 diabetes care-a systematic review and network meta-analysis., PMID:40471293
Letter: Amiodarone-Induced Thyrotoxicosis Following Semaglutide-Associated Weight Loss., PMID:40470537
Glucagon-like peptide-1 receptor agonist use prior to spinal surgery results in reduced postoperative length of stay: A propensity-score matched analysis., PMID:40470002