Catalog No.
KVV99901
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. SARS-CoV-2 RBD has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 RBD Mouse IgG present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Goat Anti-Mouse IgG antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 RBD Mouse IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 Mouse IgG Antibody
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 RBD Mouse IgG concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
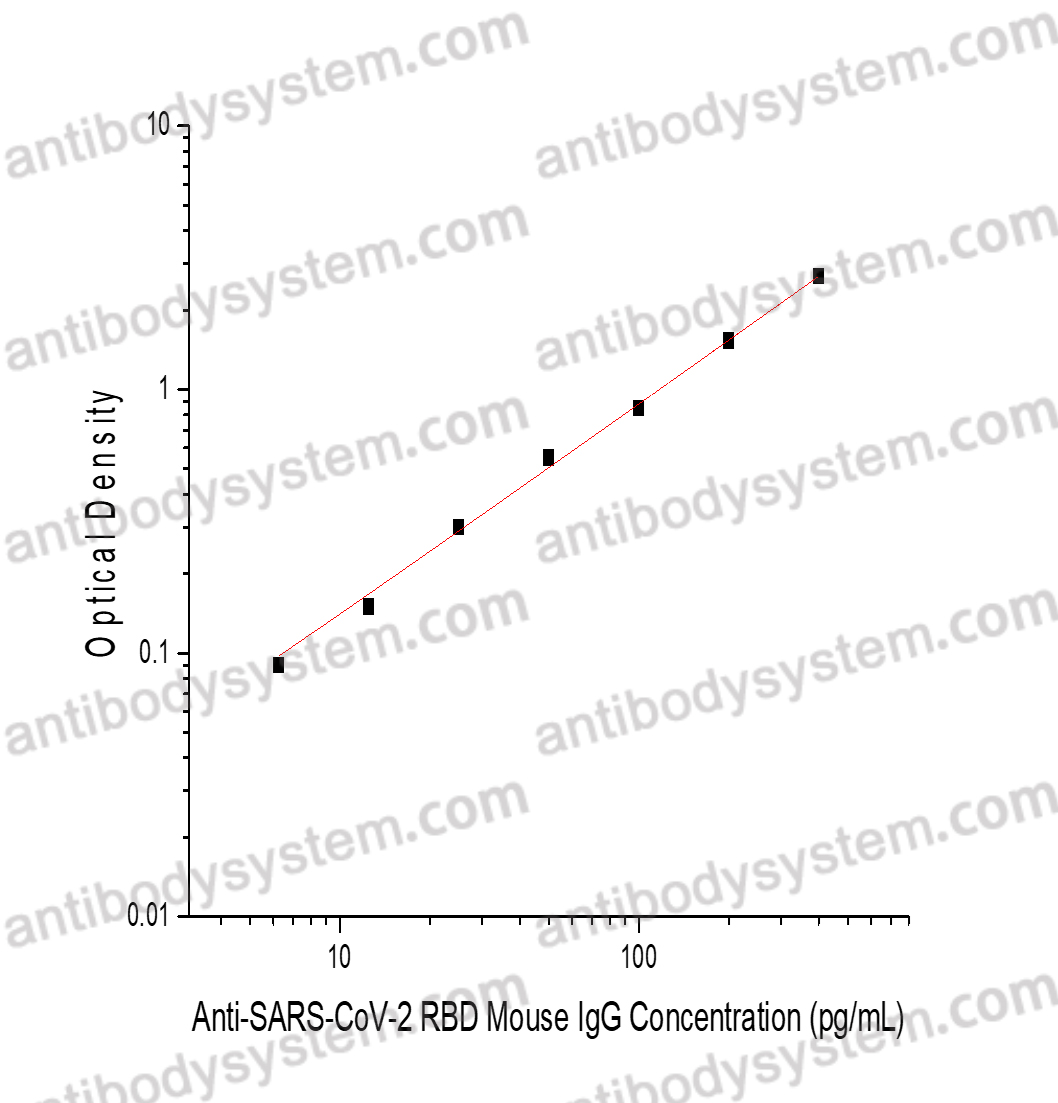
Range
6.25 - 400 pg/mL
Sensitivity
2.38 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (pg/mL)
|
181.5
|
31.1
|
7.5
|
176.8
|
29.6
|
7.2
|
|
Standard deviation
|
6.0
|
0.8
|
0.2
|
6.2
|
1.1
|
0.2
|
|
CV (%)
|
3.3
|
2.7
|
2.7
|
3.5
|
3.8
|
2.2
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Immune response induced by the recombinant novel coronavirus vaccine (Adenovirus type 5 vector) (Ad5-nCoV) in persons living with HIV (PLWH)., PMID:40498774
Detectable SARS-CoV-2 specific immune responses in recovered unvaccinated individuals 250 days post wild type infection., PMID:40498720
Determination of resilience of a panel of broadly neutralizing mAbs to emerging variants of SARS-CoV-2 generated using reverse genetics., PMID:40496810
Viral evolution prediction identifies broadly neutralizing antibodies to existing and prospective SARS-CoV-2 variants., PMID:40494884
Genetic Variations in TLR2 and TLR4 Genes and Their Association With COVID-19 Severity and Inflammatory Markers in the Moroccan Population., PMID:40483559
GWAS identifies genetic loci for antibody response to SARS-CoV-2 vaccines in patients with systemic autoimmune diseases and healthy individuals., PMID:40463545
XBB.1.5 RBD-Based Bivalent Vaccines Induced Antibody Responses Against SARS-CoV-2 Variants in Mice., PMID:40432153
Vaccine-Induced Humoral and Cellular Response to SARS-CoV-2 in Multiple Sclerosis Patients on Ocrelizumab., PMID:40432100
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Development of a self-assembling multimeric Bann-RBD fusion protein in Pichia pastoris as a potential COVID-19 vaccine candidate., PMID:40425664
Comparative study of humoral and cellular immunity against SARS-CoV-2 induced by different COVID-19 vaccine types: Insights into protection against wildtype, Delta and JN.1 omicron strains., PMID:40408899
Specific thresholds of circulating antibody titers predict against infection and reduced disease severity in SARS-CoV-2 close contacts., PMID:40405410
Immunogenicity Evaluation of a SARS-CoV-2 BA.2 Subunit Vaccine Formulated with CpG 1826 plus alum Dual Adjuvant., PMID:40383947
An RBD-Fc mucosal vaccine provides variant-proof protection against SARS-CoV-2 in mice and hamsters., PMID:40383816
Analysis of the Total Immunoglobulin G (IgG) and Its Subclasses Over Time in Coronavirus Disease 2019-Recovered Patients and Its Association With Disease Severity: A Single-Center Prospective Cohort Study., PMID:40383689
Estimating population immunity to SARS-CoV-2 by random sampling from primary and secondary healthcare in Scotland, May 2024., PMID:40381379
Safety and immunogenicity of SARS-CoV-2 protein subunit recombinant vaccine (Indovac®) in healthy populations aged 18 years and above in Indonesia: A phase I, observer-blind, randomized, controlled study., PMID:40381203
Comparative performance of serum and plasma samples in SARS-CoV-2 serology and neutralization assays., PMID:40374015
DNA-Engineered Modular Nanovaccines Featuring Precise Topology for Enhanced Immunogenicity., PMID:40371439
Сomprehensive approach to comparative characteristics of clinical and laboratory parameters of the study in children - in 6 months after Covid-19 treatment and 6 months after Covid-19 vaccination., PMID:40367469
Mimicking immune complexes for efficient antibody responses., PMID:40356891
Identifying Neurological Autoantibodies in COVID-19: mGluR2 as a Marker of Immune Dysregulation During the Omicron Outbreak in China., PMID:40343769
Regulatory cytokines modulate early isotype-specific response associated with COVID-19 survival., PMID:40342417
Plasma SARS-CoV-2 nucleocapsid antigen levels are associated with lung infection and tissue-damage biomarkers., PMID:40339608
Geographical Differences in SARS-CoV-2 Antibody Response Dynamics and Neutralisation Profiles to Mild COVID-19: Lessons from a UK-Uganda Comparison., PMID:40333205
Repeated-dose toxicity and immunogenicity evaluation of a recombinant subunit COVID-19 vaccine (ZF2001) in rats., PMID:40330020
Temporal correlations between RBD-ACE2 blocking and binding antibodies to SARS-CoV-2 variants in CoronaVac-vaccinated individuals and their persistence in COVID-19 patients., PMID:40328892
Dynamics of SARS-CoV-2 Spike Receptor-Binding Domain-Targeted Specific Peripheral Memory B Cells in Patients With End-Stage Chronic Kidney Disease Undergoing Replacement Therapy Following COVID-19 Vaccination., PMID:40326950
Minimal Impact of Prior Common Cold Coronavirus Exposure on Immune Responses to Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination or Infection Risk in Older Adults in Congregate Care., PMID:40313478
SARS-CoV-2 Seroprevalence in Indoor House Cats From the Lisbon Area During the COVID-19 Pandemic, 2019-2021., PMID:40303070
Differential immunogenicity in people living with HIV with varying CD4 levels after bivalent mRNA COVID-19 booster vaccination., PMID:40299994
Corrigendum: Anti-RBD IgG antibodies from endemic coronaviruses do not protect against the acquisition of SARS-CoV-2 infection among exposed uninfected individuals., PMID:40292279
Characterization of Nanobody Binding to Distinct Regions of the SARS-CoV-2 Spike Protein by Flow Virometry., PMID:40285013
Glycosylated Receptor-Binding-Domain-Targeting Mucosal Vaccines Protect Against SARS-CoV-2 Omicron and MERS-CoV., PMID:40266218
COVALENCE STUDY: Immunogenicity and Reactogenicity of a COVID-19 mRNA Vaccine in an Open-Label Cohort of Long-Survivor Patients with Metastatic Lung Cancer., PMID:40266144
Comparative evaluation of in-house ELISA and two commercial serological assays for the detection of antibodies against SARS-CoV-2., PMID:40263514
Recombinant receptor-binding motif of spike COVID-19 vaccine candidate induces SARS-CoV-2 neutralizing antibody response., PMID:40256231
Distinct immune memory induced by SARS-CoV-2 in convalescent liver transplant recipients., PMID:40242765
COVID-19 inactivated booster vaccines elicit strong protection against SARS-CoV-2 wild-type and Omicron variant in patients with breast cancer., PMID:40236454
Phenotypic heterogeneity defines B cell responses to repeated SARS-CoV-2 exposures through vaccination and infection., PMID:40222009
Modifying the glycosylation profile of SARS-CoV-2 spike-based subunit vaccines alters focusing of the humoral immune response in a mouse model., PMID:40217109
Spike specific IgG3 and nucleocapsid IgG response in serum serve as distinguishing immunological markers between SARS-CoV-2 infection and vaccination., PMID:40213555
The effect of COVID vaccination timing on the seroprevalence of IgG antibodies: evidence from the Guayas region of Ecuador., PMID:40201360
Neutralizing antibody responses after a two-dose regimen with BNT162b2, CoronaVac or ChAdOx1-S in Brazil: Differential neutralization of SARS-CoV-2 omicron variants., PMID:40185297
Immunogenicity and safety of monovalent and bivalent SARS-CoV-2 variant adapted RBD-based protein booster vaccines in adults previously immunized with different vaccine platforms: A phase II/III, randomized clinical trial., PMID:40179522
A replicon-based COVID-19 vaccine candidate delivered by tobacco mosaic virus-like particles., PMID:40168732
Rapid Sterilization of Clinical Apheresis Blood Products Using Ultra-High Dose Rate Radiation., PMID:40141066
Flow cytometric analysis of the SARS coronavirus 2 antibodies in human plasma., PMID:40133428
In-house assays for detecting anti-SARS-CoV-2 antibodies in serum and urine: Correlation with COVID-19 severity from a cohort study in Qatar., PMID:40117875
Impact of pre-existing immunity on humoral and cellular responses to CoronaVac in SARS-CoV-2 variants: A focus on common human Coronaviruses., PMID:40117238