Catalog No.
KAV00801
Description
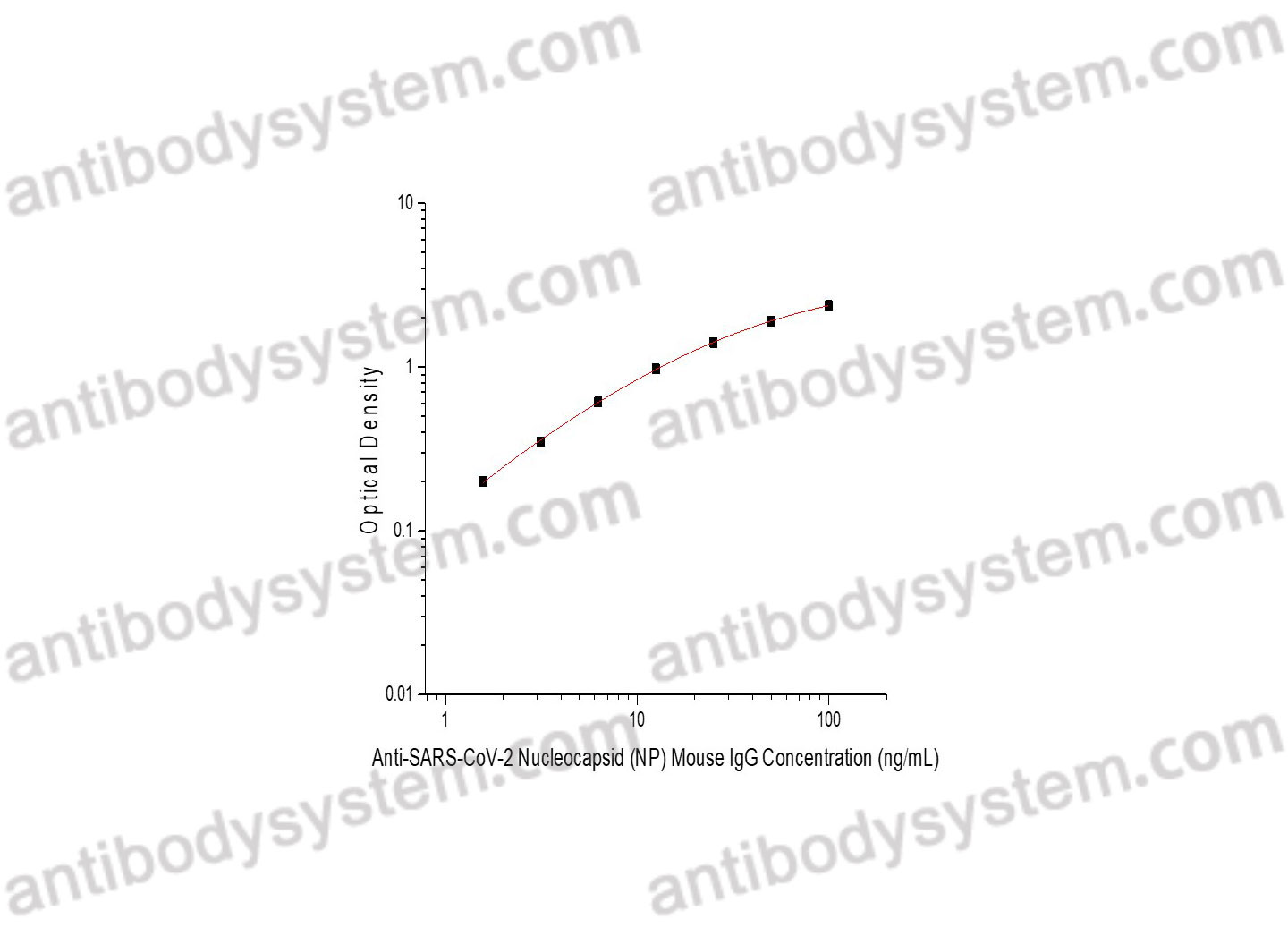
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 Nucleocapsid (NP) protein has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Nucleocapsid (NP) Mouse IgG present is bound by the immobilized protein. After washing away any unbound substances, a HRP-labeled antibody specific for Mouse IgG is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Mouse SARS-CoV-2 Nucleocapsid (NP) IgG bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 Nucleocapsid (NP) Mouse IgG Antibody
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
1.56 - 100 ng/mL
Sensitivity
0.06 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
44.3
|
12.7
|
3.0
|
44.2
|
13.3
|
3.0
|
|
Standard deviation
|
2.4
|
0.5
|
0.2
|
4.0
|
1.1
|
0.2
|
|
CV (%)
|
5.4
|
4.1
|
6.0
|
9.2
|
8.0
|
6.4
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
SARS-CoV-2 seroprevalence in pregnant women in Kilifi, Kenya from March 2020 to March 2022., PMID:38169905
Characterization of SARS-CoV-2-specific humoral immunity and associated factors in the healthy population post-vaccination., PMID:38103966
SARS-CoV-2 seroprevalence determination in pets and camels in Egypt using multispecies enzyme-linked immunosorbent assay., PMID:38061231
Kinetics of specific anti-SARS-CoV-2 IgM, IgA, and IgG responses during the first 12 months after SARS-CoV-2 infection: A prospective longitudinal study., PMID:37437051
Evaluation of SARS-CoV-2 Specific Antibodies in Recovered Patients by Different ELISA Kits., PMID:37395262
The detection of IgG class antibodies against SARS-CoV-2 nucleocapsid protein by application of nanoparticles., PMID:36808369
Accuracy of serological tests for COVID-19: A systematic review and meta-analysis., PMID:36589993
SARSPLEX: Multiplex Serological ELISA with a Holistic Approach., PMID:36560597
A Novel Dry-Stabilized Whole Blood Microsampling and Protein Extraction Method for Testing of SARS-CoV-2 Antibody Titers., PMID:36298625
SARS-CoV-2 Seroprevalence Study in Pediatric Patients and Health Care Workers Using Multiplex Antibody Immunoassays., PMID:36146844
DEVELOPMENT AND VALIDATION OF AN ENZYME-LINKED IMMUNOASSAY KIT FOR DIAGNOSIS AND SURVEILLANCE OF COVID-19., PMID:35993012
Development of robust, indigenous ELISA for detection of IgG antibodies against CoV-2 N and S proteins: mass screening., PMID:35976427
Development and validation of an enzyme-linked immunoassay kit for diagnosis and surveillance of COVID-19., PMID:35959109
Evaluation of Performance of Detection of Immunoglobulin G and Immunoglobulin M Antibody Against Spike Protein of SARS-CoV-2 by a Rapid Kit in a Real-Life Hospital Setting., PMID:35558113
Seroprevalence and immunological memory against SARS-CoV-2 in lung cancer patients: the SOLID study., PMID:35242627
Estimating SARS-CoV-2 Seroprevalence in Canadian Blood Donors, April 2020 to March 2021: Improving Accuracy with Multiple Assays., PMID:35196819
IgG antibody titers against SARS-CoV-2 nucleocapsid protein correlate with the severity of COVID-19 patients., PMID:34922455
Antibody Responses to SARS-CoV-2 Infection-Comparative Determination of Seroprevalence in Two High-Throughput Assays versus a Sensitive Spike Protein ELISA., PMID:34835241
Rapid antibody testing for SARS-CoV-2 vaccine response in pediatric healthcare workers., PMID:34601142
[Herd immunity to SARS-CoV-2 in the Novosibirsk Region population amid the COVID-19 pandemic]., PMID:34545722
Development of a Nucleocapsid Protein-Based ELISA for Detection of Human IgM and IgG Antibodies to SARS-CoV-2., PMID:33869946
Application of newly developed SARS-CoV2 serology test along with real-time PCR for early detection in health care workers and on-time plasma donation., PMID:33869895
Comparative evaluation of SARS-CoV-2 IgG assays against nucleocapsid and spike antigens., PMID:33720878
Automated Western immunoblotting detection of anti-SARS-CoV-2 serum antibodies., PMID:33660134
Development and performance evaluation of a rapid in-house ELISA for retrospective serosurveillance of SARS-CoV-2., PMID:33529223
Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays., PMID:33319585
Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak., PMID:32681308