Catalog No.
KAV00303
Description
PRINCIPLE OF THE ASSAY This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 RBD (BA.5) has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 RBD (BA.5) Human IgM present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgM antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 RBD (BA.5) Human IgM bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 RBD (BA.5) Human IgM Antibody
Applications
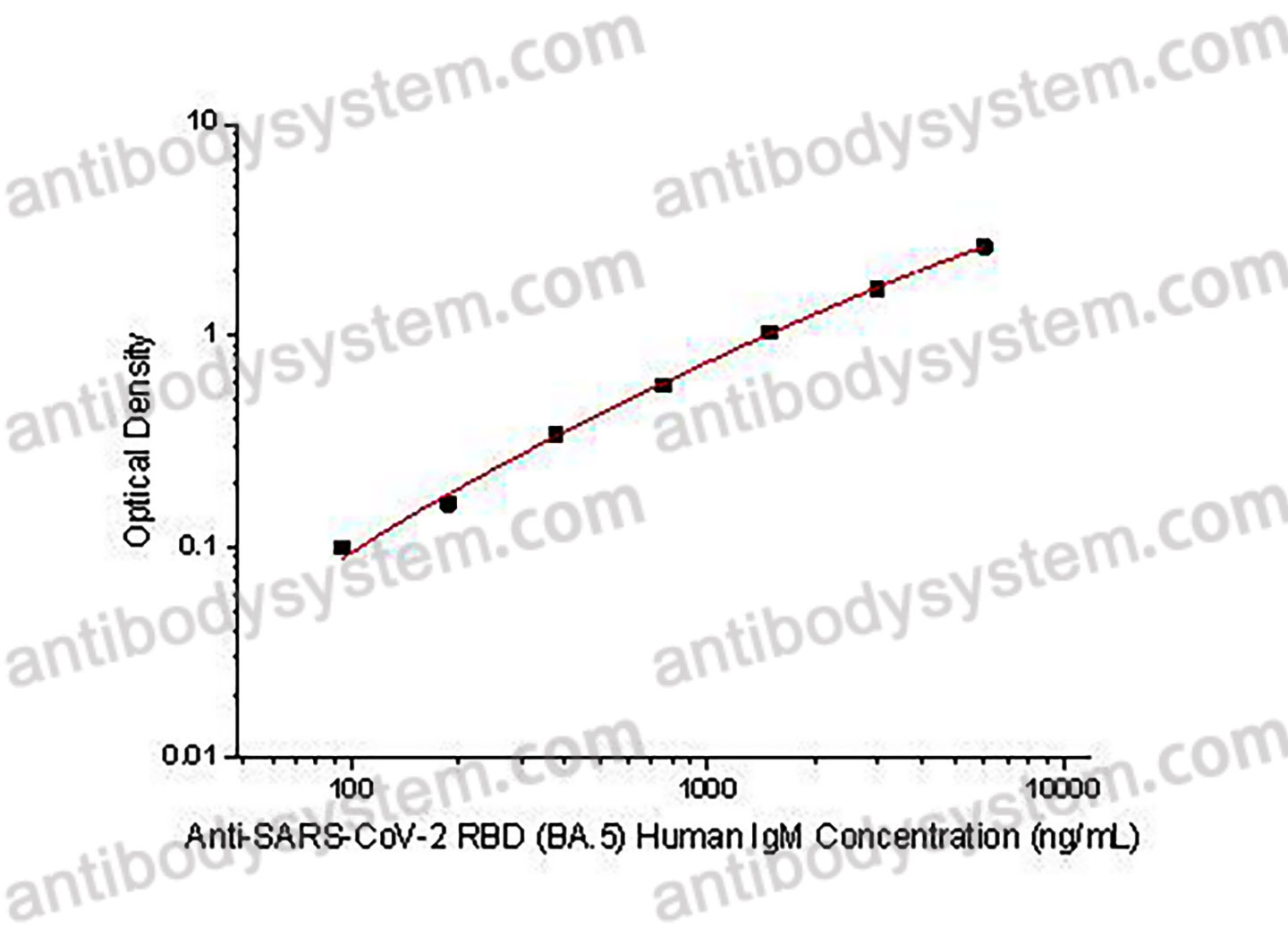
Used for the quantitative determination of Anti-SARS-CoV-2 RBD (BA.5) Human IgM concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
93.75 - 6,000 ng/mL
Sensitivity
22.06 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision |
Inter-Assay Precision |
||||
|
Sample |
1 |
2 |
3 |
1 |
2 |
3 |
|
n |
16 |
16 |
16 |
24 |
24 |
24 |
|
Mean (ng/mL) |
3734.3 |
845.0 |
213.1 |
4080.8 |
993.0 |
222.0 |
|
Standard deviation |
130.2 |
26.7 |
19.0 |
289.7 |
51.1 |
26.3 |
|
CV (%) |
3.5 |
3.2 |
8.9 |
7.1 |
5.1 |
11.8 |
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.