Catalog No.
KAV00108
Description
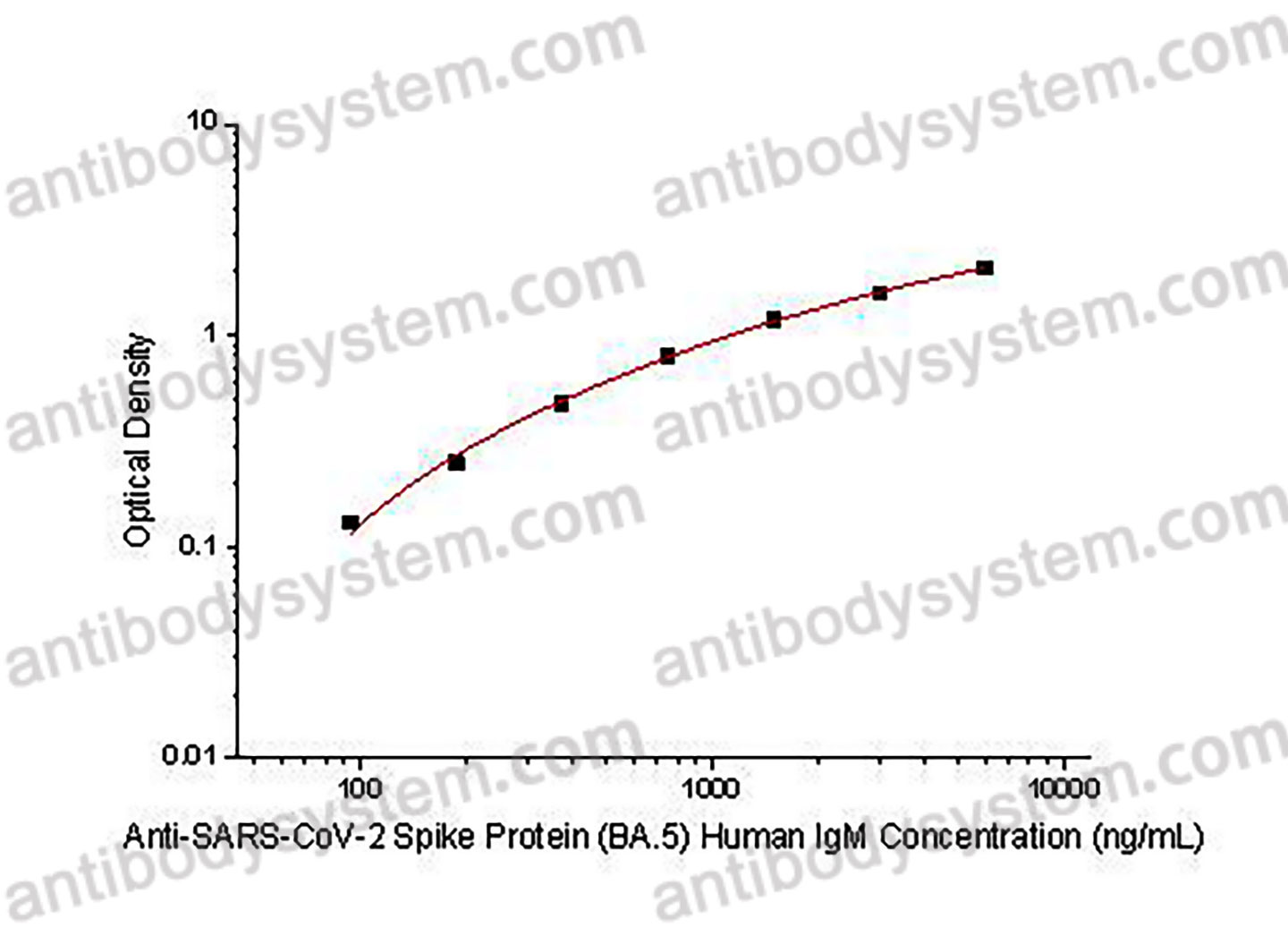
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 Spike Protein (BA.5) has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Spike Protein (BA.5) Human IgM present is bound by the immobilized protein. After washing away any unbound substances, a biotin-labeled Mouse Anti-Human IgM antibody is added to the wells. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Spike Protein (BA.5) Human IgM bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 Spike Protein (BA.5) Human IgM Antibody
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Spike Protein (BA.5) Human IgM concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
93.75 - 6,000 ng/mL
Sensitivity
50.2 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
4091.3
|
1014.0
|
242.8
|
4683.1
|
1090.5
|
215.6
|
|
Standard deviation
|
312.5
|
39.0
|
8.0
|
492.4
|
105.6
|
11.5
|
|
CV (%)
|
7.6
|
3.8
|
3.3
|
10.5
|
9.7
|
5.3
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 10% prior to the expiration date under appropriate storage condition.
Analysis of humoral and cellular immune activation up to 21 months after heterologous and homologous COVID-19 vaccination., PMID:40386778
Mimicking immune complexes for efficient antibody responses., PMID:40356891
Impact of pre-existing anti-polyethylene glycol (PEG) IgM on biodistribution and humoral response of intramuscularly administered PEGylated mRNA loaded lipid nanoparticle., PMID:40339658
Dual-screen-printed electrode platform for simultaneous detection of IgG and IgM antibodies using magnetic beads nanocomplexes., PMID:40250514
Virus-specific antibody responses in severe acute respiratory syndrome coronavirus 2-infected and vaccinated individuals., PMID:40157431
Flow cytometric analysis of the SARS coronavirus 2 antibodies in human plasma., PMID:40133428
Rapid detection of systemic and mucosal antibody responses to COVID-19 infection or vaccination., PMID:40132457
An allelic atlas of immunoglobulin heavy chain variable regions reveals antibody binding epitope preference resilient to SARS-CoV-2 mutation escape., PMID:39840032
Antibody screening-assisted multichannel nanoplasmonic sensing chip based on SERS for viral screening and variants identification., PMID:39662175
Comparable and sustained levels of S1-RBD-IgG and S1-RBD-IgA in BNT162b2 homologous and CoronaVac-BNT162b2 heterologous booster vaccination: A 22-month prospective study in Malaysia., PMID:39490114
Analytical measuring interval, linearity, and precision of serology assays for detection of SARS-CoV-2 antibodies according to CLSI guidelines., PMID:39480103
Adaptive multi-epitope targeting and avidity-enhanced nanobody platform for ultrapotent, durable antiviral therapy., PMID:39447570
Characterisation of the antibody-mediated selective pressure driving intra-host evolution of SARS-CoV-2 in prolonged infection., PMID:39405332
Relationship between HLA-II Gene Polymorphisms and Immune Response in COVID-19 Survivors and Volunteers Vaccinated against This Infection., PMID:39340619
Monitoring SARS-CoV-2 IgA, IgM and IgG antibodies in dried blood and saliva samples using antibody proximity extension assays (AbPEA)., PMID:39289450
Persistent and robust antibody responses to ChAdOx1-S Oxford-AstraZeneca (ChAdOx1-S, Covishield) SARS-CoV-2 vaccine observed in Ugandans across varied baseline immune profiles., PMID:39074077
Factors Influencing Breast Milk Antibody Titers during the Coronavirus Disease 2019 Pandemic: An Observational Study., PMID:39064762
Performance assessment of a new serological diagnostic test for COVID-19 with candidate peptides from spike and nucleocapsid viral proteins., PMID:39042245
Intranasal adenovirus-vectored Omicron vaccine induced nasal immunoglobulin A has superior neutralizing potency than serum antibodies., PMID:39039046
SARS-CoV-2 seroprevalence among Beninese pregnant women in the third year of the pandemic., PMID:38956517
Titers of IgG and IgA against SARS-CoV-2 proteins and their association with symptoms in mild COVID-19 infection., PMID:38830902
SARS-CoV-2 Omicron: Viral Evolution, Immune Evasion, and Alternative Durable Therapeutic Strategies., PMID:38793580
The single-dose Janssen Ad26.COV2.S COVID-19 vaccine elicited robust and persistent anti-spike IgG antibody responses in a 12-month Ugandan cohort., PMID:38779677
Heterologous Ad26/Ad5 adenovirus-vectored vaccines elicited SARS-CoV-2-specific antibody responses with potent Fc activities., PMID:38779671
Enhanced potency of an IgM-like nanobody targeting conserved epitope in SARS-CoV-2 spike N-terminal domain., PMID:38740785
Suppression of host humoral immunity by Borrelia burgdorferi varies over the course of infection., PMID:38514468
Evaluation of Pulmonary Function Tests, Dyspnea Scores, and Antibody Levels at the Six-Month Follow-Up of Patients Hospitalized for COVID-19 Pneumonia., PMID:38476506
A Novel Polymer Nanoparticle Polydimethyl Diallyl Ammonium Chloride as An Adjuvant Enhances the Immune Response of SARS-CoV-2 Subunit Vaccine., PMID:38436662
Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection., PMID:38342940
Detection of Specific Immunoglobulins in the Saliva of Patients With Mild COVID-19., PMID:38213933
Antibody response to Covid-19 vaccine (AstraZeneca) amongst Healthcare Workers in a Tertiary Hospital in Nigeria., PMID:38096114
Rapid increase in salivary IgA and broad recognition of spike protein following SARS-CoV-2 vaccination., PMID:38056502
Revaccination of patients with systemic lupus erythematosus or rheumatoid arthritis without an initial COVID-19 vaccine response elicits seroconversion in half of the patients., PMID:37877429
Comparison of Anti-SARS-CoV-2-Specific Antibody Signatures in Maternal and Infant Blood after COVID-19 Infection versus COVID-19 Vaccination during Pregnancy., PMID:37774748
Longitudinal anti-SARS-CoV-2 antibody immune response in acute and convalescent patients., PMID:37743860
Development of a cost-effective quantitative in-house ELISA assay for screening anti-S1 IgG antibodies targeting SARS-CoV-2., PMID:37675173
Adjuvanted-SARS-CoV-2 Spike Protein-Based Microparticulate Vaccine Delivered by Dissolving Microneedles Induces Humoral, Mucosal, and Cellular Immune Responses in Mice., PMID:37631046
[Development and preservation of specific T-cell immunity after COVID-19 or vaccination against this infection]., PMID:37436412
SARS-CoV-2 vaccine breakthrough infections (VBI) by Omicron variant (B.1.1.529) and consequences in structural and functional impact., PMID:37423342
Assessment of Broadly Reactive Responses in Patients With MERS-CoV Infection and SARS-CoV-2 Vaccination., PMID:37389876
Validity of Rapid Antibody Testing for COVID-19 Vaccine in Homeless People., PMID:37376699
Dynamics of SARS-CoV-2-Specific B Cell Memory Responses in Infected and Vaccinated Individuals., PMID:37140898
Cellular Immune Responses to SARS-CoV-2 in Exposed Seronegative Individuals., PMID:37112977
Adaptive immune response to BNT162b2 mRNA vaccine in immunocompromised adolescent patients., PMID:37051242
Total escape of SARS-CoV-2 from dual monoclonal antibody therapy in an immunocompromised patient., PMID:37037847
A new multiplex SARS-CoV-2 antigen microarray showed correlation of IgG, IgA, and IgM antibodies from patients with COVID-19 disease severity and maintenance of relative IgA and IgM antigen binding over time., PMID:36996259
Exposure of low-temperature plasma after vaccination in tongue promotes systemic IgM induction against spike protein of SARS-CoV-2., PMID:36919453
Delivery of spike-RBD by bacterial type three secretion system for SARS-CoV-2 vaccine development., PMID:36895557
Wild-type SARS-CoV-2 neutralizing immunity decreases across variants and over time but correlates well with diagnostic testing., PMID:36845123
Homologous booster immunization with an inactivated vaccine induced robust antibody response in healthcare workers: A retrospective study., PMID:36817474