Catalog No.
KVV00101
Description
PRINCIPLE OF THE ASSAY
This assay employs the quantitative indirect enzyme immunoassay technique. Recombinant SARS-CoV-2 Spike Protein has been pre-coated onto a microplate. Standards or samples are pipetted into the wells and any Anti-SARS-CoV-2 Spike Protein Mouse IgA present is bound by the immobilized protein. After washing away any unbound substances, a HRP-labeled antibody specific for Mouse IgA is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of Anti-SARS-CoV-2 Spike Protein Mouse IgA bound in the initial step. The color development is stopped and the intensity of the color is measured.
Specificity
Anti-SARS-CoV-2 Spike Protein Mouse IgA Antibody
Applications
Used for the quantitative determination of Anti-SARS-CoV-2 Spike Protein Mouse IgA concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
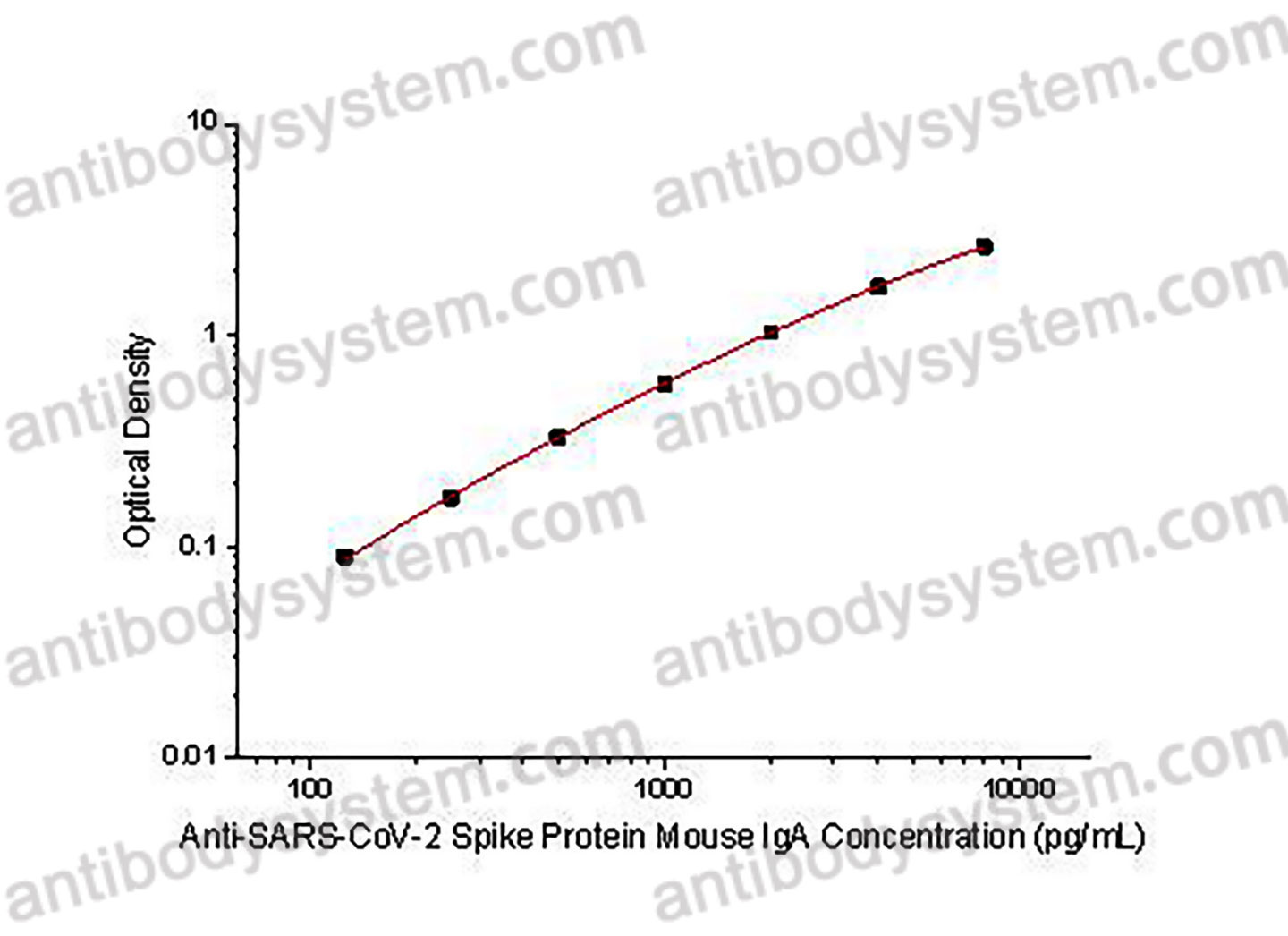
Range
125 - 8,000 pg/mL
Sensitivity
47.47 pg/mL
Precision
Intra-Assay Precision (Precision within an assay): <10%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <15%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (pg/mL)
|
3663.2
|
940.1
|
234.9
|
3794.4
|
938.8
|
230.5
|
|
Standard deviation
|
130.2
|
23.5
|
7.5
|
133.6
|
35.2
|
21.9
|
|
CV (%)
|
3.6
|
2.5
|
3.2
|
3.5
|
3.7
|
9.5
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Individuals Infected with SARS-CoV-2 Prior to COVID-19 Vaccination Maintain Vaccine-Induced RBD-Specific Antibody Levels and Viral Neutralization Activity for One Year., PMID:40431652
Construction of a variable fragment (Fv)-immunoglobulin A (IgA) anti-receptor binding domain (RBD) SARS-CoV-2 library based on IgA from Indonesian COVID-19 survivors., PMID:40403817
The Combination of TLR4 and TLR9 Agonists with Self-Amplifying RNA Lipid Nanoparticles Leads to a More Powerful Immune Response Against SARS-CoV-2., PMID:40401447
Mucosal unadjuvanted booster vaccines elicit local IgA responses by conversion of pre-existing immunity in mice., PMID:40360777
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity., PMID:40359129
Mimicking immune complexes for efficient antibody responses., PMID:40356891
Engineered bacteria as an orally administered anti-viral treatment and immunization system., PMID:40340796
Comprehensive analysis of nasal IgA antibodies induced by intranasal administration of the SARS-CoV-2 spike protein., PMID:40338637
Mucosal immune responses to SARS-CoV-2 infection and COVID-19 vaccination., PMID:40311214
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge., PMID:40258004
Nasal delivery of killed Bacillus subtilis spores protects against influenza, RSV and SARS-CoV-2., PMID:40242757
Exploring TLR agonists as adjuvants for COVID-19 oral vaccines., PMID:40184639
Study protocol for a randomised controlled trial evaluating the efficacy of dietary modulation of probiotics on nutritional status and antibody response to SARS-CoV-2 in Indonesian adolescents: gut-lung axis (DIVINE)., PMID:40180370
Immunogenicity and safety of monovalent and bivalent SARS-CoV-2 variant adapted RBD-based protein booster vaccines in adults previously immunized with different vaccine platforms: A phase II/III, randomized clinical trial., PMID:40179522
Virus-specific antibody responses in severe acute respiratory syndrome coronavirus 2-infected and vaccinated individuals., PMID:40157431
Prior SARS-CoV-2 infection affects adaptive immune responses to Omicron BA.4/BA.5 mRNA booster., PMID:40044048
Association of infection-induced antibody levels with risk of subsequent SARS-COV-2 reinfection among healthcare professionals, Rhode Island, 1 March 2020-17 February 2021., PMID:39998388
Intranasal recombinant protein subunit vaccine targeting TLR3 induces respiratory tract IgA and CD8 T cell responses and protects against respiratory virus infection., PMID:39983329
Validation and clinical performance of a non-commercial ELISA for SARS-CoV-2 anti-RBD IgA antibodies., PMID:39894142
mRNA vaccine-induced SARS-CoV-2 spike-specific IFN-γ and IL-2 T-cell responses are predictive of serological neutralization and are transiently enhanced by pre-existing cross-reactive immunity., PMID:39887249
IgA class switching enhances neutralizing potency against SARS-CoV-2 by increased antibody hinge flexibility., PMID:39828085
Oral Immunisation With Non-GMO Surface Displayed SARS-CoV-2 Spike Epitopes on Bacteria-Like Particles Provokes Robust Humoral and Cellular Immune Responses, and Modulated the Gut Microbiome in Mice., PMID:39797809
Evaluation of anti-SARS-CoV-2 RBD antibody response after booster dose of SpikoGen® in individuals with two previous doses of Sinopharm and its association with HLA-DR and -DQ alleles., PMID:39764935
A SARS-CoV-2 mucosal nanovaccine based on assembly of maltodextrin, STING agonist and polyethyleneimine., PMID:39756748
SARS-CoV-2 immune responses in patients with multiple myeloma and lenalidomide maintenance therapy., PMID:39744626
Optimizing immunogenicity and product presentation of a SARS-CoV-2 subunit vaccine composition: effects of delivery route, heterologous regimens with self-amplifying RNA vaccines, and lyophilization., PMID:39737197
Unraveling the impact of SARS-CoV-2 mutations on immunity: insights from innate immune recognition to antibody and T cell responses., PMID:39720734
Mucosal SARS-CoV-2 S1 adenovirus-based vaccine elicits robust systemic and mucosal immunity and protects against disease in animals., PMID:39629990
Comparable and sustained levels of S1-RBD-IgG and S1-RBD-IgA in BNT162b2 homologous and CoronaVac-BNT162b2 heterologous booster vaccination: A 22-month prospective study in Malaysia., PMID:39490114
Intranasal parainfluenza virus-vectored vaccine expressing SARS-CoV-2 spike protein of Delta or Omicron B.1.1.529 induces mucosal and systemic immunity and protects hamsters against homologous and heterologous challenge., PMID:39372768
Unravelling the role of secretory Immnuoglobulin-A in COVID-19: a multicentre study in nursing homes during the first wave., PMID:39354348
An intranasally administered adenovirus-vectored SARS-CoV-2 vaccine induces robust mucosal secretory IgA., PMID:39315545
Reassuring humoral and cellular immune responses to SARS-CoV-2 vaccination in participants with systemic sclerosis., PMID:39305938
The immune response to Covid-19 mRNA vaccination among Lymphoma patients receiving anti-CD20 treatment., PMID:39295862
Monitoring SARS-CoV-2 IgA, IgM and IgG antibodies in dried blood and saliva samples using antibody proximity extension assays (AbPEA)., PMID:39289450
Intranasal HD-Ad-FS vaccine induces systemic and airway mucosal immunities against SARS-CoV-2 and systemic immunity against SARS-CoV-2 variants in mice and hamsters., PMID:39281669
Post-Hoc Analysis of Potential Correlates of Protection of a Recombinant SARS-CoV-2 Spike Protein Extracellular Domain Vaccine Formulated with Advax-CpG55.2-Adjuvant., PMID:39273405
Early, Robust Mucosal Secretory Immunoglobulin A but not Immunoglobulin G Response to Severe Acute Respiratory Syndrome Coronavirus 2 Spike in Oral Fluid Is Associated With Faster Viral Clearance and Coronavirus Disease 2019 Symptom Resolution., PMID:39269503
Sequence Matters: Primary COVID-19 Vaccination after Infection Elicits Similar Anti-spike Antibody Levels, but Stronger Antibody Dependent Cell-mediated Cytotoxicity than Breakthrough Infection., PMID:39248629
Immunogenicity and efficacy of XBB.1.5 rS vaccine against the EG.5.1 variant of SARS-CoV-2 in Syrian hamsters., PMID:39230305
Mucosal adenovirus vaccine boosting elicits IgA and durably prevents XBB.1.16 infection in nonhuman primates., PMID:39227514
Salivary immune responses after COVID-19 vaccination., PMID:39226256
Intranasal Administration of Recombinant Newcastle Disease Virus Expressing SARS-CoV-2 Spike Protein Protects hACE2 TG Mice against Lethal SARS-CoV-2 Infection., PMID:39204044
Intranasal Self-Adjuvanted Lipopeptide Vaccines Elicit High Antibody Titers and Strong Cellular Responses against SARS-CoV-2., PMID:39196071
The Healthcare Study Examines the Humoral Anti-S1 Antibody Response Following mRNA Vaccination, Comparing Individuals with and without Prior SARS-CoV-2 Infection., PMID:39146978
Persistent and robust antibody responses to ChAdOx1-S Oxford-AstraZeneca (ChAdOx1-S, Covishield) SARS-CoV-2 vaccine observed in Ugandans across varied baseline immune profiles., PMID:39074077
Intranasally Inoculated SARS-CoV-2 Spike Protein Combined with Mucoadhesive Polymer Induces Broad and Long-Lasting Immunity., PMID:39066433
Factors Influencing Breast Milk Antibody Titers during the Coronavirus Disease 2019 Pandemic: An Observational Study., PMID:39064762
Performance assessment of a new serological diagnostic test for COVID-19 with candidate peptides from spike and nucleocapsid viral proteins., PMID:39042245
Intranasal adenovirus-vectored Omicron vaccine induced nasal immunoglobulin A has superior neutralizing potency than serum antibodies., PMID:39039046