Catalog No.
KDD84001
Description
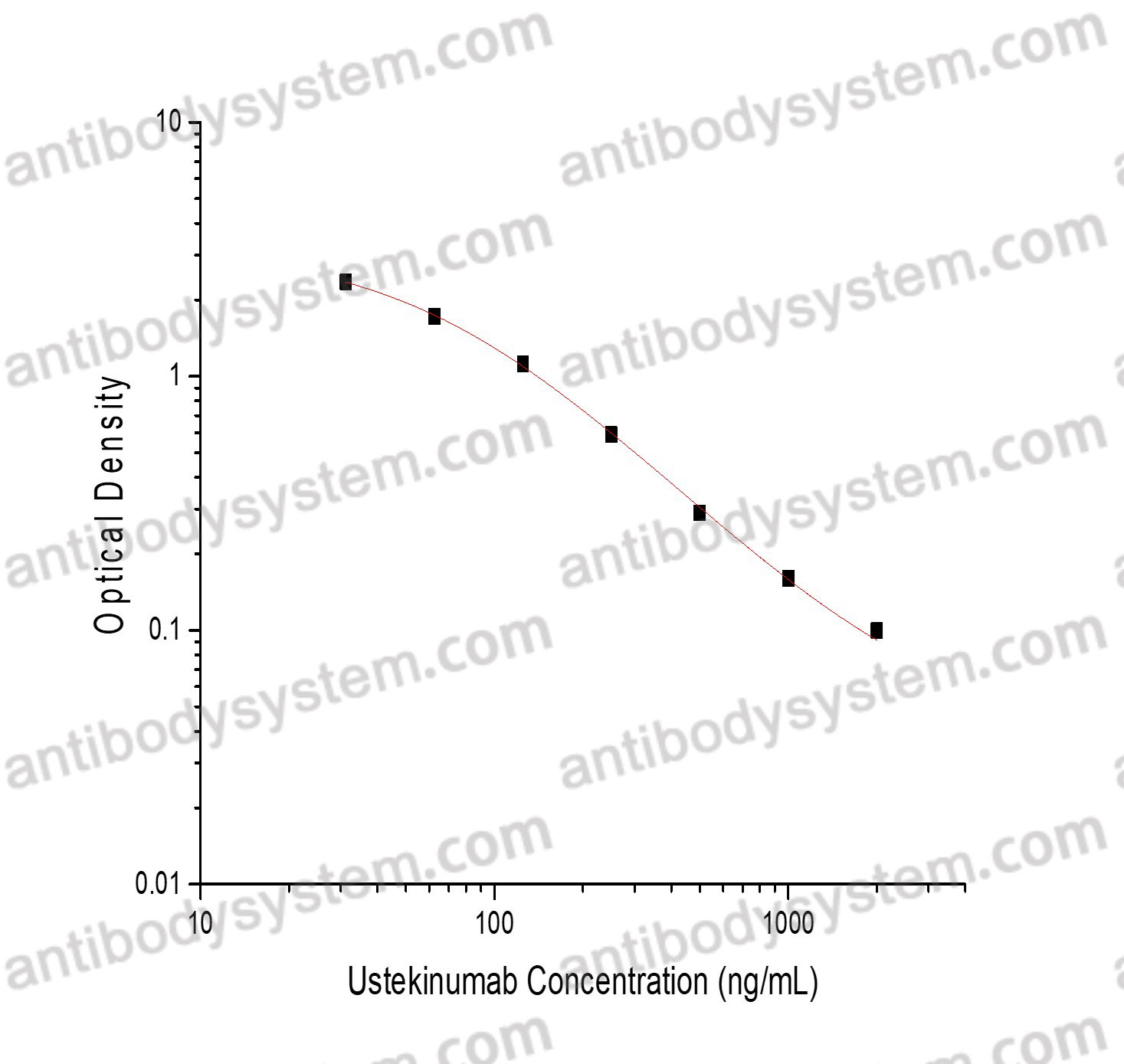
PRINCIPLE OF THE ASSAY
This assay employs the quantitative competitive enzyme immunoassay technique. Recombinant Human IL12B has been pre-coated onto a microplate. Standards or samples are premixed with biotin-labeled antibody and then pipetted into the wells. Ustekinumab in the sample competitively binds to the pre-coated protein with biotin-labeled Ustekinumab. After washing away any unbound substances, Streptavidin-HRP is added to the wells. Following a wash to remove any unbound enzyme reagent, a substrate solution is added to the wells and color develops in inversely proportion to the amount of Ustekinumab bound in the initial step. The color development is stopped and the intensity of the color is measured.
Applications
Used for the quantitative determination of Ustekinumab concentration in serum and plasma.
Detection method
Colorimetric
Sample type
Plasma, Serum
Assay type
Quantitative
Range
31.25 - 2,000 ng/mL
Sensitivity
21.40 ng/mL
Precision
Intra-Assay Precision (Precision within an assay): <20%
Three samples of known concentration were tested sixteen times on one plate to assess intra-assay precision.
Inter-Assay Precision (Precision between assays): <20%
Three samples of known concentration were tested in twenty four separate assays to assess inter-assay precision.
|
|
Intra-Assay Precision
|
Inter-Assay Precision
|
|
Sample
|
1
|
2
|
3
|
1
|
2
|
3
|
|
n
|
16
|
16
|
16
|
24
|
24
|
24
|
|
Mean (ng/mL)
|
1587.6
|
334.0
|
68.7
|
910.9
|
274.3
|
57.2
|
|
Standard deviation
|
152.5
|
29.3
|
6.5
|
106.8
|
37.7
|
10.3
|
|
CV (%)
|
9.6
|
8.8
|
9.4
|
11.7
|
13.8
|
17.9
|
Recovery
80-120%
Shipping
2-8 ℃
Stability and Storage
When the kit was stored at the recommended temperature for 6 months, the signal intensity decreased by less than 20%.
Alternative Names
CNTO 1275, TT-20, CAS: 815610-63-0
Effectiveness and Safety of Advanced Dual-Targeted Therapy in Refractory Perianal Crohn's Disease., PMID:40504122
Comparative effectiveness of ustekinumab versus infliximab in the management of perianal fistulizing Crohn's disease: a retrospective study in China., PMID:40499579
The Effectiveness of second-and-third line biologics in perianal Crohn's disease - a multicenter propensity score-matched study., PMID:40490896
Wells syndrome: emerging triggers and treatments- an updated systematic review., PMID:40488888
Metabolism and Response to Stress Gene Signatures Reveal Ulcerative Colitis Heterogeneity and Identify Patients With Increased Response to Therapy., PMID:40488582
Impact of Obesity on the Long-Term Outcomes of Advanced Therapies in IBD: A Real-World Study in Taiwan., PMID:40487282
Impact of immediate drug optimization of ustekinumab on medium term targets in Crohn's disease: results from the multicentre retrospective real-life study MUST., PMID:40484747
Safety of guselkumab and ustekinumab treatment in patients with moderate-to-severe plaque psoriasis combined with latent tuberculosis or inactive hepatitis B virus infection: A retrospective multicenter observational study., PMID:40482822
Treatment sequences, outcomes, healthcare utilization, and costs in patients with inflammatory bowel diseases requiring advanced treatment-real world comparative effectiveness from German claims data., PMID:40481399
Interleukin-23 Inhibitors for Inflammatory Bowel Disease: Pivotal Trials and Practical Considerations., PMID:40465057
Guselkumab (Tremfya) - an IL-23 antagonist for Crohn's disease., PMID:40459404
Cancer Incidence in Patients with Ulcerative Colitis Naïve to or Treated with Thiopurine and Targeted Therapies- a cohort study 2007 to 2022 with comparison to the general population., PMID:40455688
Ustekinumab Dose Optimization in Ulcerative Colitis: Is More Always Better?, PMID:40447982
Cross-phenotype genome-wide association study supports shared genetic etiology between skin and gastrointestinal tract diseases., PMID:40441863
Systematic review of comparative studies on emerging psoriasis treatments: comparing biologics with biologics, small molecule inhibitors with small molecule inhibitors, and biologics with small molecule inhibitors., PMID:40439875
Drug survival of IL-23 and IL-17 inhibitors versus other biologics for psoriasis: A British Association of Dermatologists Biologics and Immunomodulators Register cohort study., PMID:40439435
Comparative Efficacy of Ustekinumab and Guselkumab in Improving Itch in Severe Psoriasis Patients., PMID:40432363
Advanced Therapies for Inflammatory Bowel Disease and Risk of Skin Cancer: What's New?, PMID:40427207
The Effects of the Biological Agents Infliximab, Vedolizumab, and Ustekinumab on Intestinal Anastomosis: An Experimental Study in Rats., PMID:40426907
Efficacy and safety of dual-targeted therapy for refractory inflammatory bowel disease: a retrospective case series from three tertiary general hospitals in China., PMID:40421289
Predictors of drug survival of biologics in hidradenitis suppurativa: A systematic review and meta-analysis., PMID:40419221
Short-Term Effectiveness of Ustekinumab in Crohn's Disease: Results from a Real-World Retrospective Multicenter Study in China., PMID:40417421
Use of ustekinumab as the treatment of choice in patient with corticodependent immune-mediated colitis secondary to pembrolizumab., PMID:40409592
Ustekinumab in the Treatment of Crohn's Disease-A Narrative Review on Clinical Efficacy and Safety Profile., PMID:40407511
Evaluating changes in baseline characteristics and drug utilisation pattern in patients with moderate-to-severe psoriasis: findings from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) Cohort., PMID:40402160
Complete Resolution of Pityriasis Rubra Pilaris With Targeted Treatment: A Case Report., PMID:40400847
The Incidence and Management of TNF-α Inhibitor Induced Paradoxical Psoriasis in Children With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis., PMID:40400050
The 'totality of evidence' and 'extrapolation' of SB17, a ustekinumab biosimilar., PMID:40396611
Systematic review and meta-analysis of the efficacy of biologic and targeted synthetic therapies in sarcoidosis., PMID:40393718
Risk of adverse events of psoriasis treatment with biologic agents and new small molecules-BIOBADADERM Registry., PMID:40387427
A systematic review of tumor necrosis factor-α blockers, anti-interleukins, and small molecule inhibitors for dissecting cellulitis of the scalp treatment., PMID:40383754
Induction with upadacitinib in Crohn's disease: real-world experience from an early-access program in Greece., PMID:40371210
Predicting ustekinumab treatment response in Crohn's disease using pre-treatment biopsy images., PMID:40366737
Real-World Insights From Türkiye: Biologic DMARDs Usage in Spondyloarthritis Patients With Chronic Kidney Disease., PMID:40358366
Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease., PMID:40357993
Cost per remission for mirikizumab versus ustekinumab for moderately to severely active ulcerative colitis treatment from the United States commercial payer perspective., PMID:40351121
Innate and Adaptive Immunity is not Impacted by Inflammatory Bowel Disease Medications in Pregnant Women and Their Offspring., PMID:40349212
Alpha Fail: Ustekinumab to the Rescue After TNFα Failure in Patients with Moderate to Severe Crohn's Disease., PMID:40347351
Second-line strategies after anti-TNF failure in chronically active, moderate-to-severe ulcerative colitis: a retrospective, multicentre cohort study., PMID:40346848
Pyoderma Gangrenosum: A Retrospective Study Comparing TNF-α Inhibitors with Ustekinumab., PMID:40339789
Pharmacokinetic equivalence and comparative safety, tolerability, and immunogenicity of Biocon's ustekinumab (Bmab-1200) with EU-approved and US-licensed reference ustekinumab in healthy subjects: results from the Study to Test pharmacokinetic BioEquivalence of BiosimiLar ustekinumab to SteLARa (STELLAR-1)., PMID:40331766
Ustekinumab is effective in the treatment of linear psoriasis: a case report and literature review., PMID:40330485
The Effectiveness of Medical Therapies for Joint, Skin and Eye Extraintestinal Manifestations in IBD-An Umbrella Review., PMID:40329548
The real-world effectiveness of ustekinumab in patients with ulcerative colitis in the United States., PMID:40327500
One-Year Efficacy of Guselkumab Versus Advanced Therapies for the Treatment of Moderately to Severely Active Crohn's Disease: A Network Meta-Analysis., PMID:40327280
Mirikizumab (Omvoh) - an IL-23 antagonist for Crohn's disease., PMID:40324965
Prevalence of opportunistic infections in Syrian inflammatory bowel disease patients on biologic therapy: a multi-center retrospective cross-sectional study., PMID:40320559
Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study., PMID:40319105
Immunopathological and microbial signatures of inflammatory bowel disease in partial RAG deficiency., PMID:40314722