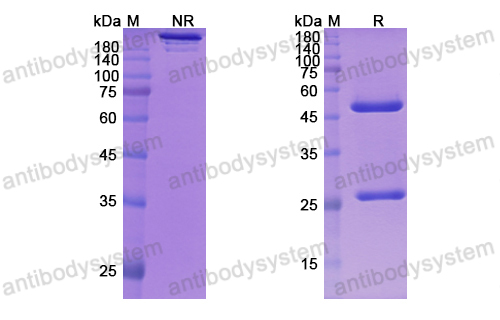
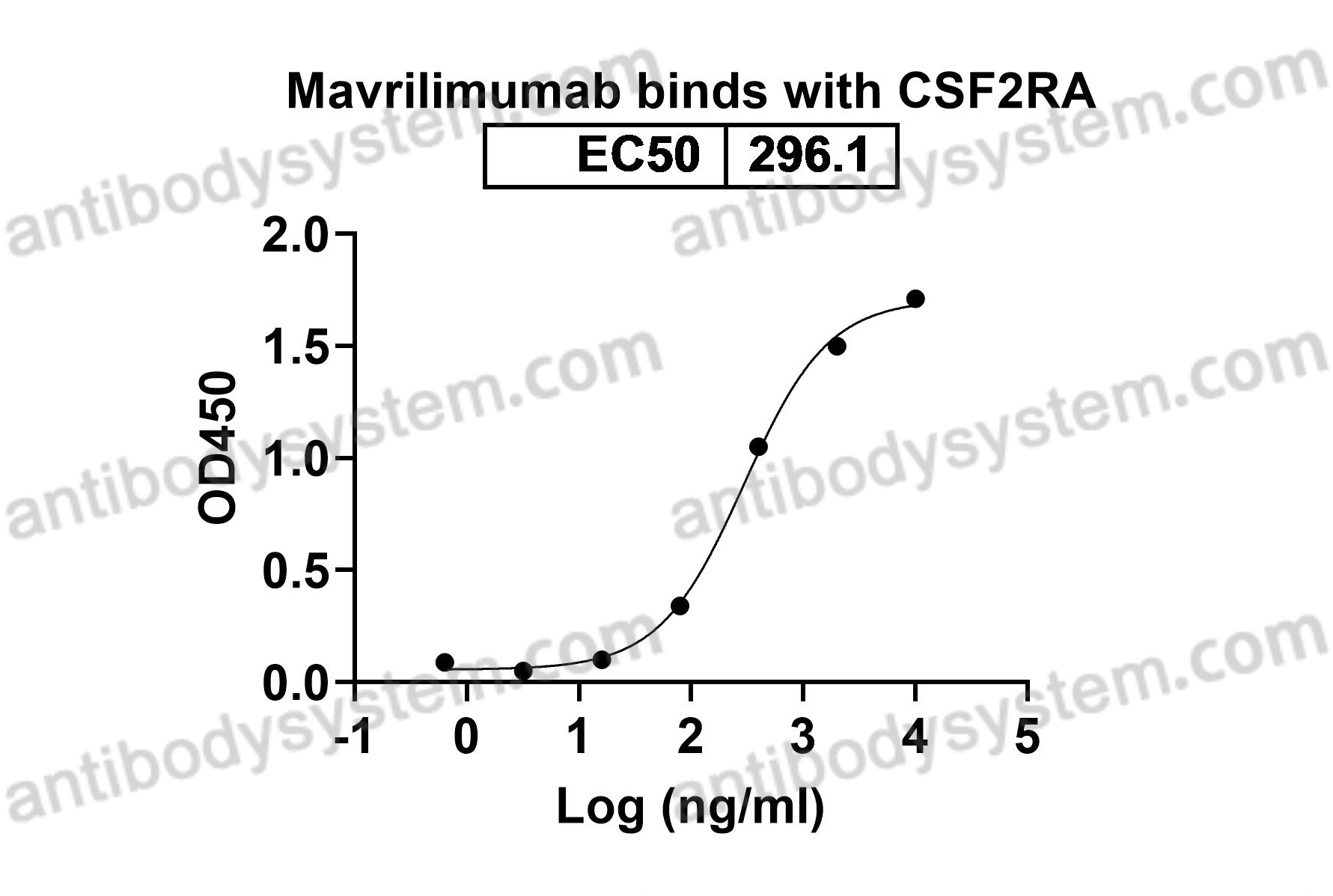
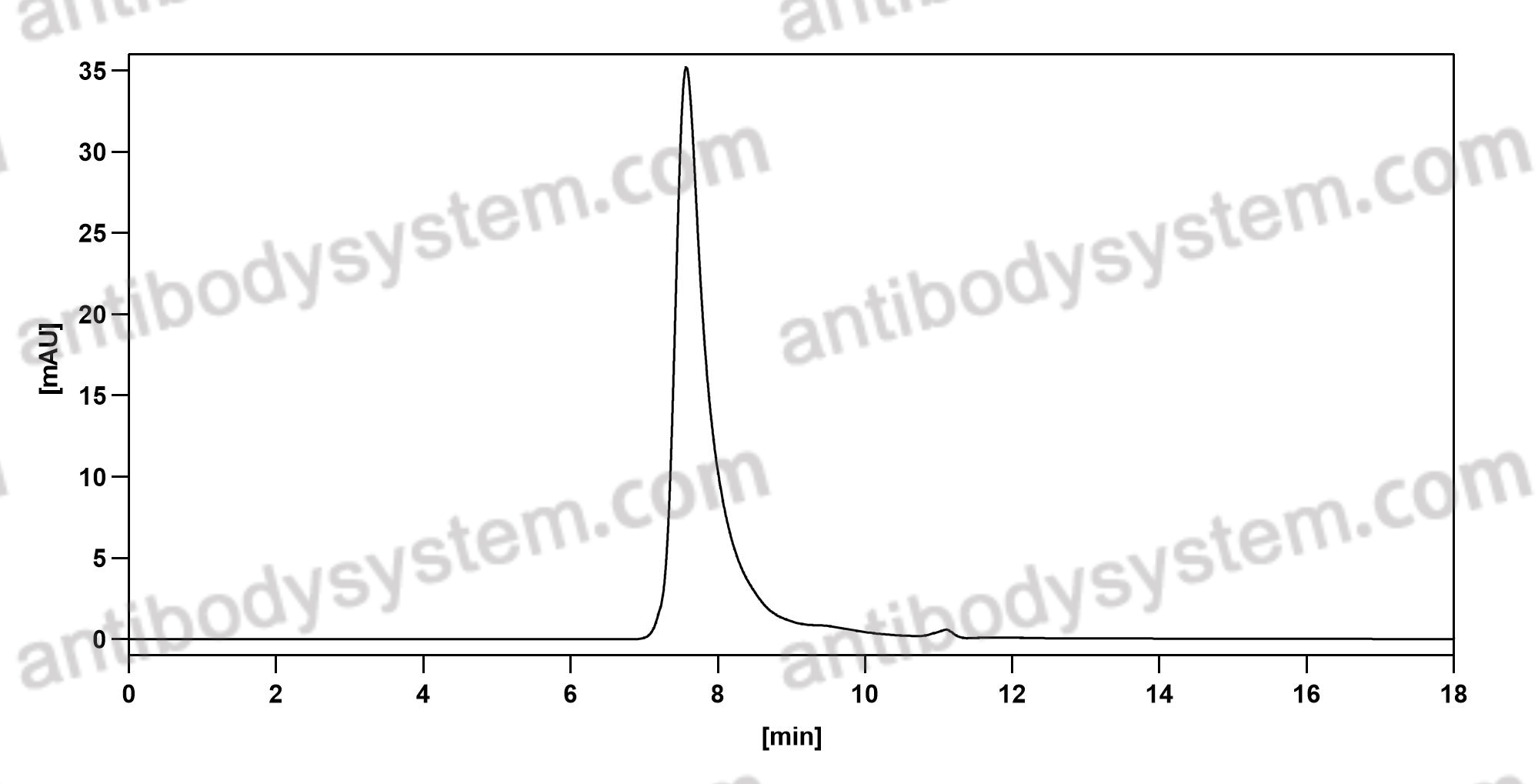
GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study, PMID: 32835256
Mavrilimumab: a unique insight and update on the current status in the treatment of rheumatoid arthritis, PMID: 31208237
Mavrilimumab: an evidence based review of its potential in the treatment of rheumatoid arthritis, PMID: 24648832
Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies, PMID: 32719685
Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor α Monoclonal Antibody: Long-Term Safety and Efficacy in Patients With Rheumatoid Arthritis, PMID: 29361199
Spotlight on mavrilimumab for the treatment of rheumatoid arthritis: evidence to date, PMID: 28144129
Investigational therapies targeting the granulocyte macrophage colony-stimulating factor receptor-α in rheumatoid arthritis: focus on mavrilimumab, PMID: 29387176
Rheumatoid arthritis: Long-term mavrilimumab safe and effective, PMID: 29416133
Mavrilimumab, a human monoclonal GM-CSF receptor-α antibody for the management of rheumatoid arthritis: a novel approach to therapy, PMID: 23094973
Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review, PMID: 33068263
Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis, PMID: 32033937
A Randomized Phase IIb Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis, PMID: 28941039
Rheumatoid arthritis: new monoclonal antibodies, PMID: 29771256
Mavrilimumab for severe COVID-19, PMID: 33521662
Mavrilimumab for severe COVID-19, PMID: 33521661
Mavrilimumab for severe COVID-19 - Authors' reply, PMID: 33521663
Pharmacodynamic biomarkers and differential effects of TNF- and GM-CSF-targeting biologics in rheumatoid arthritis, PMID: 30358109
Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study, PMID: 21613310
Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects, PMID: 31486005
Safety and Efficacy of Mavrilimumab For Rheumatoid Arthritis: A Systematic Review and Meta-Analysis, PMID: 33185165
Model-Based Discovery and Development of Biopharmaceuticals: A Case Study of Mavrilimumab, PMID: 28836356
Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis, PMID: 23234647
A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis, PMID: 28213566
Effectiveness of Mavrilimumab in Viral Infections Including SARS-CoV-2 Infection - A Brief Review, PMID: 34409778
Nonclinical safety of mavrilimumab, an anti-GMCSF receptor alpha monoclonal antibody, in cynomolgus monkeys: relevance for human safety, PMID: 24937321
Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial, PMID: 33754144
Efficacy and safety of mavrilimumab in Japanese subjects with rheumatoid arthritis: findings from a Phase IIa study, PMID: 24720551
Comparison of the efficacy and safety of tofacitinib and mavrilimumab in patients with active rheumatoid arthritis: A Bayesian network meta-analysis of randomized controlled trials, PMID: 33860755
Mechanistic modeling of antigen sink effect for mavrilimumab following intravenous administration in patients with rheumatoid arthritis, PMID: 21947370
Blockade of GM-CSF pathway induced sustained suppression of myeloid and T cell activities in rheumatoid arthritis, PMID: 29069507
The VICM biomarker is released from activated macrophages and inhibited by anti-GM-CSFRα-mAb treatment in rheumatoid arthritis patients, PMID: 30418117
Targeting GM-CSF in rheumatoid arthritis, PMID: 27586802
GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential, PMID: 25748625
GM-CSF as a therapeutic target in inflammatory diseases, PMID: 23933508
Innate and Adaptive Immunity in Giant Cell Arteritis, PMID: 33717054
Pulmonary pharmacodynamics of an anti-GM-CSFRα antibody enables therapeutic dosing that limits exposure in the lung, PMID: 27560702
Novel targeted therapies: the future of rheumatoid arthritis? Mavrilumab and tabalumab as examples, PMID: 23625979
Evaluation of Antibody Properties and Clinically Relevant Immunogenicity, Anaphylaxis, and Hypersensitivity Reactions in Two Phase III Trials of Tralokinumab in Severe, Uncontrolled Asthma, PMID: 30649752
Market watch: Upcoming market catalysts in Q4 2015, PMID: 26338153
Quantitative measurement of the target-mediated internalization kinetics of biopharmaceuticals, PMID: 25208874
Giant cell arteritis: what is new in the preclinical and early clinical development pipeline?, PMID: 34106030
Granulocyte macrophage colony stimulating factor in virus-host interactions and its implication for immunotherapy., PMID:39755463
Network-Based In Silico Analysis of New Combinations of Modern Drug Targets with Methotrexate for Response-Based Treatment of Rheumatoid Arthritis., PMID:38003865
Giant Cell Arteritis and Polymyalgia Rheumatica: Treatment Approaches and New Targets., PMID:37331730
Recent advances in the diagnosis and therapy of large vessel vasculitis., PMID:35699647
Treatment of Giant Cell Arteritis (GCA)., PMID:35407411
Advances in the Treatment of Giant Cell Arteritis., PMID:35329914
Efficacy and safety of mavrilimumab in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial., PMID:35264321
Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review., PMID:35126138
Blocking GM-CSF receptor α with mavrilimumab reduces infiltrating cells, pro-inflammatory markers and neoangiogenesis in ex vivo cultured arteries from patients with giant cell arteritis., PMID:35045965
The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations: Lessons Learned From Major Clinical Studies., PMID:34867318
Chronic pharmacological antagonism of the GM-CSF receptor in mice does not replicate the pulmonary alveolar proteinosis phenotype but does alter lung surfactant turnover., PMID:34778899
Effectiveness of Mavrilimumab in Viral Infections Including SARS-CoV-2 Infection - A Brief Review., PMID:34409778
Giant cell arteritis: what is new in the preclinical and early clinical development pipeline?, PMID:34106030
Comparison of the efficacy and safety of tofacitinib and mavrilimumab in patients with active rheumatoid arthritis: A Bayesian network meta-analysis of randomized controlled trials., PMID:33860755
Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial., PMID:33754144
Innate and Adaptive Immunity in Giant Cell Arteritis., PMID:33717054
Mavrilimumab for severe COVID-19 - Authors' reply., PMID:33521663
Mavrilimumab for severe COVID-19., PMID:33521662
Mavrilimumab for severe COVID-19., PMID:33521661
Safety and Efficacy of Mavrilimumab For Rheumatoid Arthritis: A Systematic Review and Meta-Analysis., PMID:33185165
Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review., PMID:33068263
GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study., PMID:32835256
Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies., PMID:32719685
Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis., PMID:32033937
Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects., PMID:31486005
Mavrilimumab: a unique insight and update on the current status in the treatment of rheumatoid arthritis., PMID:31208237
Evaluation of Antibody Properties and Clinically Relevant Immunogenicity, Anaphylaxis, and Hypersensitivity Reactions in Two Phase III Trials of Tralokinumab in Severe, Uncontrolled Asthma., PMID:30649752
The VICM biomarker is released from activated macrophages and inhibited by anti-GM-CSFRα-mAb treatment in rheumatoid arthritis patients., PMID:30418117
Pharmacodynamic biomarkers and differential effects of TNF- and GM-CSF-targeting biologics in rheumatoid arthritis., PMID:30358109
Rheumatoid arthritis: new monoclonal antibodies., PMID:29771256
Rheumatoid arthritis: Long-term mavrilimumab safe and effective., PMID:29416133
Investigational therapies targeting the granulocyte macrophage colony-stimulating factor receptor-α in rheumatoid arthritis: focus on mavrilimumab., PMID:29387176
Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor α Monoclonal Antibody: Long-Term Safety and Efficacy in Patients With Rheumatoid Arthritis., PMID:29361199
Blockade of GM-CSF pathway induced sustained suppression of myeloid and T cell activities in rheumatoid arthritis., PMID:29069507
A Randomized Phase IIb Study of Mavrilimumab and Golimumab in Rheumatoid Arthritis., PMID:28941039
Model-Based Discovery and Development of Biopharmaceuticals: A Case Study of Mavrilimumab., PMID:28836356
A randomised phase IIb study of mavrilimumab, a novel GM-CSF receptor alpha monoclonal antibody, in the treatment of rheumatoid arthritis., PMID:28213566
Spotlight on mavrilimumab for the treatment of rheumatoid arthritis: evidence to date., PMID:28144129
Targeting GM-CSF in rheumatoid arthritis., PMID:27586802
Pulmonary pharmacodynamics of an anti-GM-CSFRα antibody enables therapeutic dosing that limits exposure in the lung., PMID:27560702
Market watch: Upcoming market catalysts in Q4 2015., PMID:26338153
GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential., PMID:25748625
Quantitative measurement of the target-mediated internalization kinetics of biopharmaceuticals., PMID:25208874
Nonclinical safety of mavrilimumab, an anti-GMCSF receptor alpha monoclonal antibody, in cynomolgus monkeys: relevance for human safety., PMID:24937321
Efficacy and safety of mavrilimumab in Japanese subjects with rheumatoid arthritis: findings from a Phase IIa study., PMID:24720551
Mavrilimumab: an evidence based review of its potential in the treatment of rheumatoid arthritis., PMID:24648832
GM-CSF as a therapeutic target in inflammatory diseases., PMID:23933508
Novel targeted therapies: the future of rheumatoid arthritis? Mavrilumab and tabalumab as examples., PMID:23625979
Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis., PMID:23234647
Mavrilimumab, a human monoclonal GM-CSF receptor-α antibody for the management of rheumatoid arthritis: a novel approach to therapy., PMID:23094973