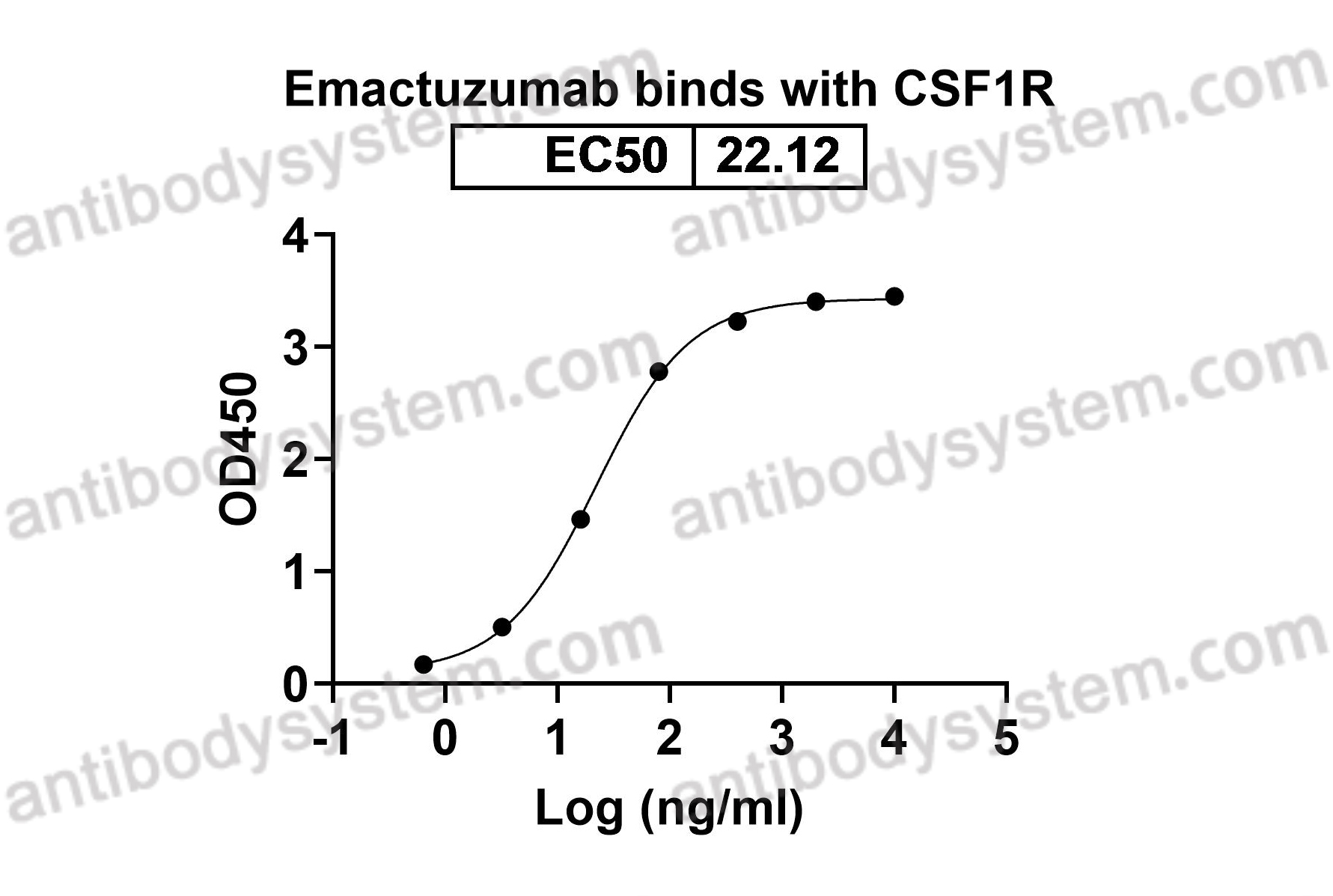
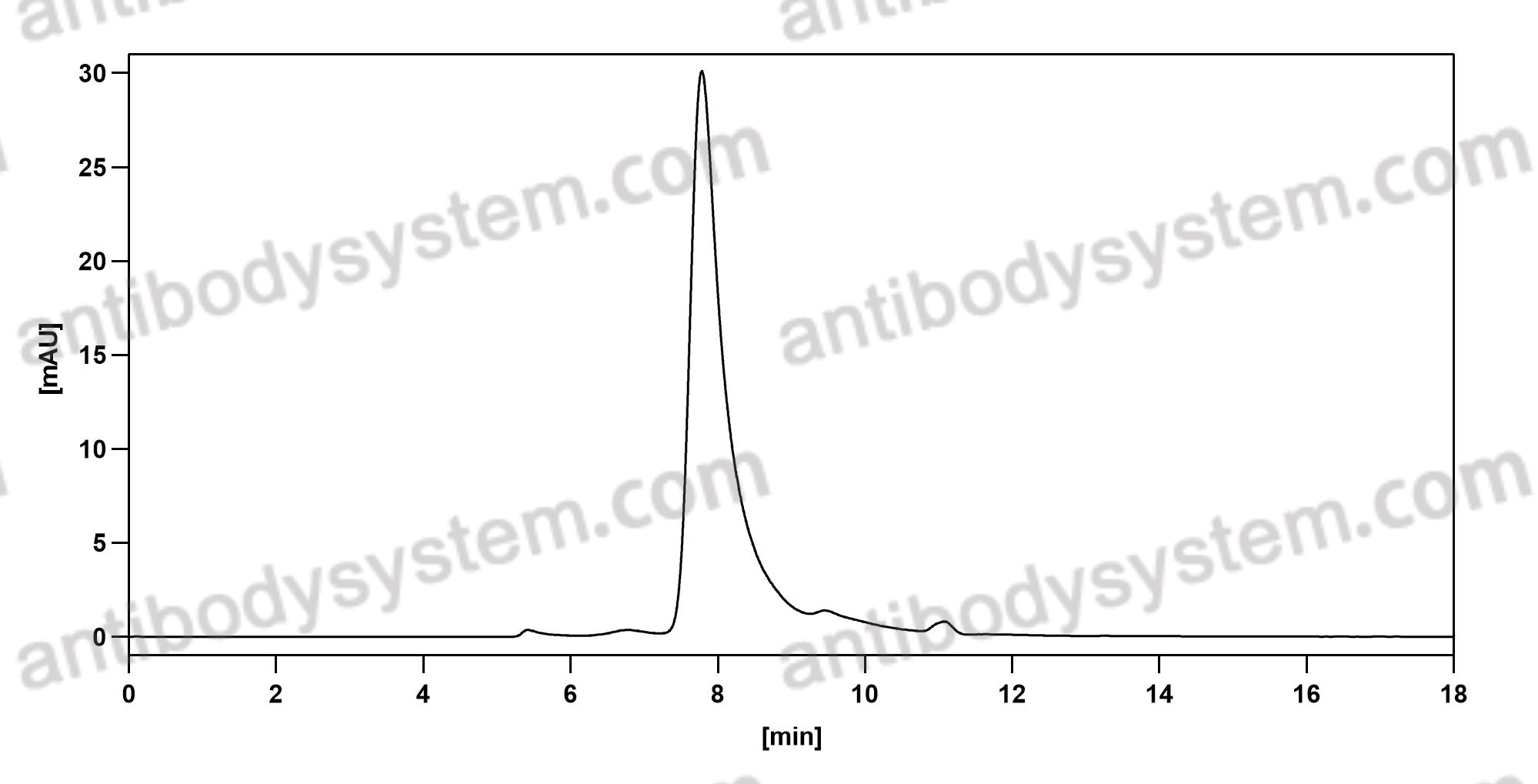
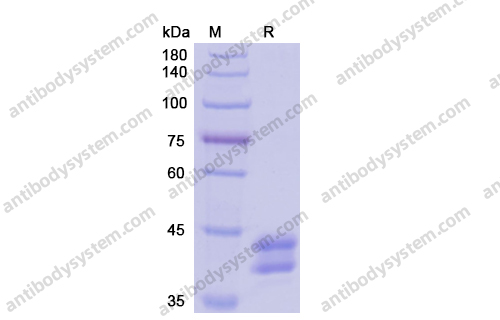
Catalog No.
DHC25202
Expression system
Mammalian Cells
Species reactivity
Human
Host species
Humanized
Isotype
IgG1-kappa
Clonality
Monoclonal
Target
CD115, CSF-1 receptor, CSF1R, M-CSF-R, CSF-1-R, Proto-oncogene c-Fms, Macrophage colony-stimulating factor 1 receptor, CSF-1R, FMS
Concentration
1 mg/ml
Endotoxin level
Please contact with the lab for this information.
Purity
>95% as determined by SDS-PAGE.
Purification
Protein A/G purified from cell culture supernatant.
Accession
P07333
Applications
Research Grade Biosimilar
Form
Liquid
Storage buffer
0.01M PBS, pH 7.4.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C short term (1-2 weeks). Store at -20°C 12 months. Store at -80°C long term.
Alternative Names
RG 7155, RO5509554, CAS: 1448221-67-7
Clone ID
Emactuzumab
Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages, PMID: 31114846
PK/PD Mediated Dose Optimization of Emactuzumab, a CSF1R Inhibitor, in Patients With Advanced Solid Tumors and Diffuse-Type Tenosynovial Giant Cell Tumor, PMID: 32575160
Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients, PMID: 33097612
Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy, PMID: 24898549
Periorbital oedema secondary to emactuzumab treated with topical 2.5% phenylephrine, PMID: 30859687
Macrophage Susceptibility to Emactuzumab (RG7155) Treatment, PMID: 27582524
Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour, PMID: 33161240
Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response, PMID: 30886344
T cell-induced CSF1 promotes melanoma resistance to PD1 blockade, PMID: 29643229
CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study, PMID: 26179200
Therapeutically-induced stable disease in oncology early clinical trials, PMID: 32470048
Macrophages at the crossroads of anticancer strategies, PMID: 31136979
Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis, PMID: 26820289
Potential contribution of tumor-associated slan + cells as anti-CSF-1R targets in human carcinoma, PMID: 28951423
Effects of IL-10 and T h 2 cytokines on human Mφ phenotype and response to CSF1R inhibitor, PMID: 29345363
Long term term follow-up of tyrosine kinase inhibitors treatments in inoperable or relapsing diffuse type tenosynovial giant cell tumors (dTGCT), PMID: 32433669
Macrophage depletion induces edema through release of matrix-degrading proteases and proteoglycan deposition, PMID: 34135110
Medical Management of Tenosynovial Giant Cell Tumor., PMID:40392406
Updates on the Treatment of Tenosynovial Giant Cell Tumor., PMID:37363972
Extensile Anterior and Posterior Knee Exposure for Complete Synovectomy of Diffuse Tenosynovial Giant Cell Tumor (Pigmented Villonodular Synovitis)., PMID:36741035
Treatment updates on tenosynovial giant cell tumor., PMID:35837703
Anti-CSF-1R emactuzumab in combination with anti-PD-L1 atezolizumab in advanced solid tumor patients naïve or experienced for immune checkpoint blockade., PMID:35577503
Macrophage depletion induces edema through release of matrix-degrading proteases and proteoglycan deposition., PMID:34135110
Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour., PMID:33161240
Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients., PMID:33097612
PK/PD Mediated Dose Optimization of Emactuzumab, a CSF1R Inhibitor, in Patients With Advanced Solid Tumors and Diffuse-Type Tenosynovial Giant Cell Tumor., PMID:32575160
Therapeutically-induced stable disease in oncology early clinical trials., PMID:32470048
Long term term follow-up of tyrosine kinase inhibitors treatments in inoperable or relapsing diffuse type tenosynovial giant cell tumors (dTGCT)., PMID:32433669
Macrophages at the crossroads of anticancer strategies., PMID:31136979
Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages., PMID:31114846
Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response., PMID:30886344
Periorbital oedema secondary to emactuzumab treated with topical 2.5% phenylephrine., PMID:30859687
T cell-induced CSF1 promotes melanoma resistance to PD1 blockade., PMID:29643229
Effects of IL-10 and Th 2 cytokines on human Mφ phenotype and response to CSF1R inhibitor., PMID:29345363
Potential contribution of tumor-associated slan+ cells as anti-CSF-1R targets in human carcinoma., PMID:28951423
Macrophage Susceptibility to Emactuzumab (RG7155) Treatment., PMID:27582524
Current Systemic Treatment Options for Tenosynovial Giant Cell Tumor/Pigmented Villonodular Synovitis: Targeting the CSF1/CSF1R Axis., PMID:26820289
CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study., PMID:26179200
Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy., PMID:24898549