Catalog No.
YHC87401
Expression system
E. coli
Species
Homo sapiens (Human)
Protein length
Met1-Ile290
Predicted molecular weight
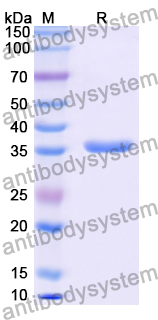
35.73 kDa
Nature
Recombinant
Endotoxin level
Please contact with the lab for this information.
Purity
>90% as determined by SDS-PAGE.
Accession
P11245
Applications
ELISA, Immunogen, SDS-PAGE, WB, Bioactivity testing in progress
Form
Lyophilized
Storage buffer
Lyophilized from a solution in PBS pH 7.4, 0.02% NLS, 1mM EDTA, 4% Trehalose, 1% Mannitol.
Reconstitution
Reconstitute in sterile water for a stock solution. A copy of datasheet will be provided with the products, please refer to it for details.
Shipping
In general, proteins are provided as lyophilized powder/frozen liquid. They are shipped out with dry ice/blue ice unless customers require otherwise.
Stability and Storage
Use a manual defrost freezer and avoid repeated freeze thaw cycles. Store at 2 to 8°C for frequent use. Store at -20 to -80°C for twelve months from the date of receipt.
Alternative Names
Arylamine N-acetyltransferase 2, 2.3.1.5, Arylamide acetylase 2, N-acetyltransferase type 2, NAT-2, Polymorphic arylamine N-acetyltransferase, PNAT, NAT2, AAC2
NAT2 Acetylation Status Predicts Hepatotoxicity During Antituberculosis Therapy: Cumulative Risk Analysis of a Multiethnic Cohort., PMID:40332508
Optimized Sampling Strategies for Isoniazid in East Asian Pediatric Populations Using Population Pharmacokinetics-Informed Approaches., PMID:40330817
Xenobiotic metabolizing gene variants and the risk of male infertility - A systematic review, meta-analysis and in silico analysis., PMID:40271533
Metabolism and genotoxicity of 4,4'-oxydianiline is dependent on N-acetyltransferase 2 genetic polymorphism., PMID:40246217
Allele-specific detection of isoniazid metabolism modulating variants of N-acetyltransferase 2 enzyme and their frequencies in the Bangladeshi population., PMID:40204038
One-year mortality of tuberculosis patients on isoniazid-based treatment and its association with rapid acetylator NAT2 genotypes., PMID:40147587
Pharmacogenomic heterogeneity of N-acetyltransferase 2: a comprehensive analysis of real world data in Indian tuberculosis patients and from literature and database review., PMID:40138446
Association of NAT2 promoter hypermethylation with susceptibility to hepatotoxicity due to antituberculosis drugs and biomarker potential., PMID:40133601
NAT2 activity increases cytotoxicity of anthracycline antibiotics and HDAC inhibitors., PMID:40081304
Pharmacogenetics of plasma dolutegravir exposure during 1-month rifapentine/isoniazid treatment of latent tuberculosis., PMID:39960813
Genetic polymorphisms and anti-tuberculosis drug-induced liver injury: an umbrella review of the evidence., PMID:39954223
The role of isoniazid dosage and NAT2 gene polymorphism in the treatment of tuberculous meningitis., PMID:39902038
Pharmacogenomic profiling of variants affecting efficacy and toxicity of anti-infective medicines in a south Asian population from Sri Lanka., PMID:39893405
Metabolic effects of heterocyclic amines on insulin‑induced AKT phosphorylation and gluconeogenic gene expression are modified by N -acetyltransferase 2 genetic polymorphism., PMID:39878101
Characterization of NAT, GST, and CYP2E1 Genetic Variation in Sub-Saharan African Populations: Implications for Treatment of Tuberculosis and Other Diseases., PMID:39829327
Effect of Genetic Variants on Rosuvastatin Pharmacokinetics in Healthy Volunteers: Involvement of ABCG2, SLCO1B1 and NAT2., PMID:39796117
Organic Sunscreens-Is Their Placenta Permeability the Only Issue Associated with Exposure During Pregnancy? In Silico Studies of Sunscreens' Placenta Permeability and Interactions with Selected Placental Enzymes., PMID:39769924
Clinical predictors of 3-month isoniazid rifapentine (3HP)-related adverse drug reactions (ADR) during tuberculosis preventive therapy (PAnDoRA-3HP study): an observational study protocol., PMID:39740953
The N-Acetyltransferase 2 Polymorphism and Susceptibility to Inflammatory Bowel Disease: A Case-Control Study., PMID:39720855
Pharmacogenomic insights into tuberculosis treatment shows the NAT2 genetic variants linked to hepatotoxicity risk: a systematic review and meta-analysis., PMID:39639188
Spermidine mediates acetylhypusination of RIPK1 to suppress diabetes onset and progression., PMID:39511379
Pharmacogenetics of tuberculosis treatment toxicity and effectiveness in a large Brazilian cohort., PMID:39470346
N-Acetyltransferase 2 gene polymorphism and its serum levels in vitiligo patients., PMID:39404470
Differential distribution of NAT2 polymorphisms and NAT2 acetylator phenotypes among indigenous populations of the Brazilian Amazon., PMID:39259702
Precision Medicine Strategies to Improve Isoniazid Therapy in Patients with Tuberculosis., PMID:39153028
Drug-induced hepatotoxicity and association with slow acetylation variants NAT2*5 and NAT2*6 in Cameroonian patients with tuberculosis and HIV co-infection., PMID:39085767
Pharmacogenetic Study of Drugs Affecting Mycobacterium tuberculosis., PMID:38916393
N-acetyltransferase metabolism and DNA damage following exposure to 4,4'-oxydianiline in human bronchial epithelial cells., PMID:38906436
LINC00520 promotes colorectal cancer progression through miRNA-195-3p / NAT2 axis., PMID:38836661
Pharmacogenetic variability of tuberculosis biomarkers in native and mestizo Peruvian populations., PMID:38666760
Genetic and clinical predictors of rifapentine and isoniazid pharmacokinetics in paediatrics with tuberculosis infection., PMID:38661209
Effect of Food Composition on the PK of Isoniazid Quantitatively Explained Using Physiologically Based Biopharmaceutics Modeling., PMID:38658473
Cascading Detection of Hydrogen Sulfide and N-Acetyltransferase 2 in Hepatocellular Carcinoma Cells Using a Two-Photon Fluorescent Probe., PMID:38657082
Efficacy, safety, and pharmacokinetics of isoniazid affected by NAT2 polymorphisms in patients with tuberculosis: A systematic review., PMID:38629592
High-Dose Isoniazid Lacks EARLY Bactericidal Activity against Isoniazid-resistant Tuberculosis Mediated by katG Mutations: A Randomized Phase II Clinical Trial., PMID:38564365
Model-Informed Precision Dosing of Isoniazid: Parametric Population Pharmacokinetics Model Repository., PMID:38500691
Synergistic toxicity with copper contributes to NAT2-associated isoniazid toxicity., PMID:38424191
The Arylamine N-Acetyltransferases as Therapeutic Targets in Metabolic Diseases Associated with Mitochondrial Dysfunction., PMID:38351074
Polymorphisms in drug-metabolizing genes and urinary bladder cancer susceptibility and prognosis: Possible impacts and future management., PMID:38350514
Pharmacogenetic Analysis of an 8-Year Old Girl with Reye Syndrome Associated with Use of Naproxen., PMID:38311849
Influence of N-acetyltransferase 2 polymorphisms and clinical variables on liver function profile of tuberculosis patients., PMID:38287694
Prediction Models for Adverse Drug Reactions During Tuberculosis Treatment in Brazil., PMID:38262629
Towards pharmacogenomics-guided tuberculosis (TB) therapy: N-acetyltransferase-2 genotypes among TB-infected Kenyans of mixed ethnicity., PMID:38184575
Metabolism-Related Prognostic Biomarkers, Purine Metabolism and Anti-Tumor Immunity in Colon Adenocarcinoma., PMID:38179743
Arylamines N-acetyltransferases 1 and 2: physiology, genetics and epigenetics., PMID:40000435
Direct Comparative Analysis of a Pharmacogenomics Panel with PacBio Hifi® Long-Read and Illumina Short-Read Sequencing., PMID:38138882
Relevance of NAT2 genotype and clinical factors to risk for antituberculosis drug-induced liver injury., PMID:38131213
WGCNA combined with machine learning to find potential biomarkers of liver cancer., PMID:38115320
Association of the cytochrome P450 and arylamine N-acetyltransferase gene polymorphisms with the incidence of head and neck cancer in Polish population., PMID:38099560
Risk adjustment model for tuberculosis compared to non-tuberculosis mycobacterium or latent tuberculosis infection: Center for Personalized Precision Medicine of Tuberculosis (cPMTb) cohort database., PMID:38001469