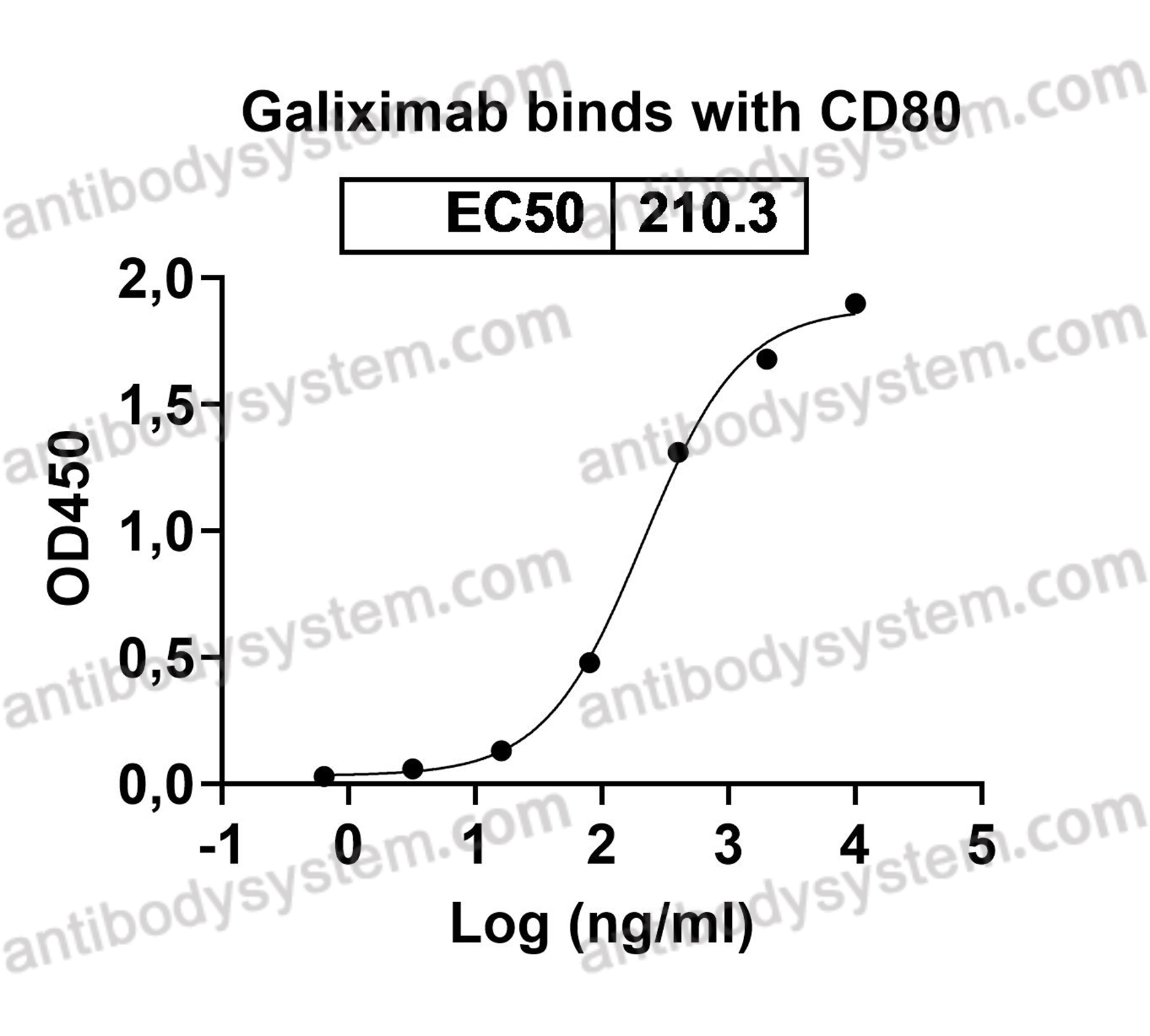
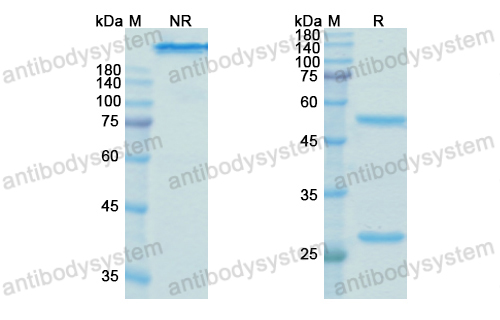
Galiximab: a review, PMID: 20092425
The use of galiximab in non-Hodgkin lymphoma, PMID: 18854281
Galiximab signals B-NHL cells and inhibits the activities of NF-κB-induced YY1- and snail-resistant factors: mechanism of sensitization to apoptosis by chemoimmunotherapeutic drugs, PMID: 22267549
Galiximab (anti-CD80)-induced growth inhibition and prolongation of survival in vivo of B-NHL tumor xenografts and potentiation by the combination with fludarabine, PMID: 23764770
Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma, PMID: 15994148
Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness, PMID: 29390097
The anti-CD80 primatized monoclonal antibody, galiximab, is well-tolerated but has limited activity in relapsed Hodgkin lymphoma: Cancer and Leukemia Group B 50602 (Alliance), PMID: 23194022
A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma, PMID: 17470451
Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness, PMID: 22357442
Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis, PMID: 15093549
Galiximab in relapsed hodgkin lymphoma, PMID: 21317862
Initial trials of anti-CD80 monoclonal antibody (Galiximab) therapy for patients with relapsed or refractory follicular lymphoma, PMID: 12672278
Newer monoclonal antibodies for hematological malignancies, PMID: 18565392
Emerging drugs for chronic lymphocytic leukaemia, PMID: 16503834
Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma, PMID: 17335294
Gateways to clinical trials, PMID: 16357953
Gateways to clinical trials, PMID: 15319815
Gateways to clinical trials, PMID: 12851663
Beyond rituximab: The future of monoclonal antibodies in B-cell non-Hodgkin lymphoma, PMID: 20425465
The prognostic significance of PFS24 in follicular lymphoma following firstline immunotherapy: A combined analysis of 3 CALGB trials, PMID: 30575311
Discontinued drugs 2010: rheumatology, allergy and dermatology, pulmonary, PMID: 21793791
Recombinantly engineered human proteins: transforming the treatment of psoriasis, PMID: 12482385
The use of monoclonal antibodies in the treatment of autoimmune complications of chronic lymphocytic leukemia, PMID: 23667725
CD80 Insights as Therapeutic Target in the Current and Future Treatment Options of Frequent-Relapse Minimal Change Disease, PMID: 33506028
Gateways to clinical trials. March 2003, PMID: 12731460
CD80 (B7.1) is expressed on both malignant B cells and nonmalignant stromal cells in non-Hodgkin lymphoma, PMID: 22076940
IDEC-114 (IDEC), PMID: 11569938
Combination treatment approaches and novel therapies for lymphoma, PMID: 18154235
Association of rapamycin and co-stimulation blockade using anti-B7 antibodies in renal allotransplantation in baboons, PMID: 15069178
Clinical and histologic response to single-dose treatment of moderate to severe psoriasis with an anti-CD80 monoclonal antibody, PMID: 12399760
Classification updates and clinical investigations in indolent non-Hodgkin lymphoma, PMID: 18998258
Immunotherapy in indolent Non-Hodgkin's Lymphoma., PMID:35663281
CD80 Insights as Therapeutic Target in the Current and Future Treatment Options of Frequent-Relapse Minimal Change Disease., PMID:33506028
The prognostic significance of PFS24 in follicular lymphoma following firstline immunotherapy: A combined analysis of 3 CALGB trials., PMID:30575311
Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness., PMID:29390097
Galiximab (anti-CD80)-induced growth inhibition and prolongation of survival in vivo of B-NHL tumor xenografts and potentiation by the combination with fludarabine., PMID:23764770
The use of monoclonal antibodies in the treatment of autoimmune complications of chronic lymphocytic leukemia., PMID:23667725
The anti-CD80 primatized monoclonal antibody, galiximab, is well-tolerated but has limited activity in relapsed Hodgkin lymphoma: Cancer and Leukemia Group B 50602 (Alliance)., PMID:23194022
Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus rituximab (CALGB 50402): Follicular Lymphoma International Prognostic Index (FLIPI) score is predictive of upfront immunotherapy responsiveness., PMID:22357442
Galiximab signals B-NHL cells and inhibits the activities of NF-κB-induced YY1- and snail-resistant factors: mechanism of sensitization to apoptosis by chemoimmunotherapeutic drugs., PMID:22267549
CD80 (B7.1) is expressed on both malignant B cells and nonmalignant stromal cells in non-Hodgkin lymphoma., PMID:22076940
Discontinued drugs 2010: rheumatology, allergy and dermatology, pulmonary., PMID:21793791
Galiximab in relapsed hodgkin lymphoma., PMID:21317862
Galiximab: a review., PMID:20092425
Classification updates and clinical investigations in indolent non-Hodgkin lymphoma., PMID:18998258
The use of galiximab in non-Hodgkin lymphoma., PMID:18854281
Beyond rituximab: The future of monoclonal antibodies in B-cell non-Hodgkin lymphoma., PMID:20425465
Newer monoclonal antibodies for hematological malignancies., PMID:18565392
Combination treatment approaches and novel therapies for lymphoma., PMID:18154235
A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma., PMID:17470451
Monoclonal antibodies in the treatment of non-Hodgkin's lymphoma., PMID:17335294
Emerging drugs for chronic lymphocytic leukaemia., PMID:16503834
Gateways to clinical trials., PMID:16357953
Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma., PMID:15994148
Gateways to clinical trials., PMID:15319815
Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis., PMID:15093549
Association of rapamycin and co-stimulation blockade using anti-B7 antibodies in renal allotransplantation in baboons., PMID:15069178
Gateways to clinical trials., PMID:12851663
Gateways to clinical trials. March 2003., PMID:12731460
Initial trials of anti-CD80 monoclonal antibody (Galiximab) therapy for patients with relapsed or refractory follicular lymphoma., PMID:12672278
Recombinantly engineered human proteins: transforming the treatment of psoriasis., PMID:12482385
Clinical and histologic response to single-dose treatment of moderate to severe psoriasis with an anti-CD80 monoclonal antibody., PMID:12399760
IDEC-114 (IDEC)., PMID:11569938